The endangered status of Patella ferruginea in a limpet assemblage hotspot (Plane Island: South West Alboran, Mediterranean Sea): Local distribution factors and species abundance
Laboratoire du Réseau de surveillance environnemental (LRSE), Nature & Life Sciences Faculty, Oran1 University, Algeria mus.kallouche@gmail.com
Laboratoire du Réseau de surveillance environnemental (LRSE), Nature & Life Sciences Faculty, Oran1 University, Algeria
Laboratoire du Réseau de surveillance environnemental (LRSE), Nature & Life Sciences Faculty, Oran1 University, Algeria
Laboratoire du Réseau de surveillance environnemental (LRSE), Nature & Life Sciences Faculty, Oran1 University, Algeria
Laboratoire du Réseau de surveillance environnemental (LRSE), Nature & Life Sciences Faculty, Oran1 University, Algeria
Nature & Life Sciences Faculty, Oran1 University, Algeria
Laboratoire du Réseau de surveillance environnemental (LRSE), Nature & Life Sciences Faculty, Oran1 University, Algeria
Abstract
Limpets constitute an important key species in the intertidal zone. The limpets’ assemblage is evaluated in Plane Island (Paloma Island, Algerian West coast) by detailed censuses which were performed in 8 zones in the main island and 9 islets. Results allowed the estimation of a total population of 3993 of the endangered Patella ferruginea (1.78ind/m), 1861 P. rustica (0.82ind/m), and 383 of the blue Mediterranean limpet P. caerulea (0.17 ind/m). The ferruginous limpet distribution around Plane Island was influenced by abiotic factors as hydrodynamics and the sea current direction. Conversely, anthropogenic factors had an important impact despite the island's distance from the mainland. This study finds that in the face of global warming, the island surface will be reduced, however limpets will have an important contribution to the island's areas and its conservation of biodiversity.
Keywords
Limpets, assemblage, abiotic factors, Patella ferruginea, densities
Introduction
Taxonomic and trophic assemblage structure differs between regions to determine the importance of habitat features as structuring drivers of these assemblages (Andrades et al., 2018). Limpets constitute an important taxonomic group of the rocky shore Intertidal Mediterranean zones (Tlig et al., 2010). This group of Molluscan Gastropod Prosobranchia belongs to the Patellidae Family (Grande et al., 2008). The genus Patella is a grazing species performing its activity by night. They are favoured by their organization which allows them to resist wave shocks and desiccation thanks to the power of their adherence (Branche, 1981; Holmes et al., 2002; Boudouresque, 2005; Boudouresque and Bianchi, 2013). This adherence can be observed in both natural and artificial substratum (Frankiel and Moueza, 1982; Kallouche et al., 2011; Kallouche et al., 2014; Kallouche, 2018).
Along the Algerian coasts, the genus Patella constitue an important population (Beldi et al., 2012, Kallouche et al., 2014a). Many limpet species are present along the Algerian coast. Some of them are largely distibuted: Patella rustica, P. caerulea and P. ferruginea and others are rarely present: P. ulyssiponensis, P. depressa and P. vulgata. The false limpet Siphonaria pectinata is also present (Kallouche et al., 2014a). This species seems to belong to Patellidae family, however its breathing system is different (Gofas et al., 2011). Grande et al. (2008) classifies it among the opistobranches in view of genetic similarities. In order to characterise Algerian limpets, some molecular studies about genetic variability were recently performed in Algerian coasts (Kallouche et al., 2018; Bouzaza and Mezali, 2018).
The ferruginous limpet is a species endemic to the Western Mediterranean Basin. It is the most endangered marine invertebrate — listed in the European Council Directive 92/43/EEC on the conservation of natural habitat of wild fauna and flora — and needs strict protection (Ramos, 1998). This species is currently at high risk of extinction (Laborel-Deguen and Laborel, 1991a; Templado and Moreno, 1997). Patella ferruginea is included in the annexes of endangered or threatened species of Barcelona and Bern Conventions and in the European Habitat Directive (Templado et al., 2004). In Algeria, it is declared as protected species in the official journal of Algerian republic (2006).
For these reasons, much research about biology, ecology and distribution has been undertaken with regard to this gastropod since the 1970s (Ghisotti and Melone, 1970; Curini-Galletti, 1979; Granfils, 1982; Laborel-Deguen, 1985; Biagi and Poli., 1986; Boudouresque and Laborel-Deguen, 1986; Laborel-Daguen and Laborel 1990 and 1991a,b; Porcheddu and Milella 1991; Moreno, 1992; Laborel-Deguen et al., 1993; Templado, 1996 and 1997; Cretella et al., 1994; Aparici-Seguer et al., 1995; Boumaza and Semroud, 2001; Paralcuellos et al., 2003; Machordom et al., 2010; Espinosa et al., 2005, 2006, 2008; Guallart et al., 2006a,b, 2010, 2011, 2012 and 2013a,b,c; Tlig et al., 2010; Guallart and Espinosa 2012, 2016), and even before in Algerian coasts by Pallary (1900) and Laurent (1939) with the first citations.
Many authors report the highest densities of the ferruginous limpet in North African insular zones, and protected marine areas: Chafarinas Islands (Guallart et al., 2016), Ceuta (Riviera-Ingraham et al., 2011), Alboran Island (CMA-JA, 2014) in Spain (North Africa), Zambra and Zambretta islands (Zarrouk et al., 2018) in Tunisia. In Algeria the most important densities are reported in Rachgoun Island (Taibi et al., 2014), Habibas Island (Boumaza et Semroud, 2001; Espinosa, 2009; Larbi-Doukara, 2019); the main work about Plane Island ferruginous limpets was done by Espinosa (2009). Thus, only two sectors were studied; and it is not enough representative of all the insular zone.
Patella ferruginea is known by its important densities in North African islands compared to other species. In this work, the population of this limpet is evaluated in the whole stretch coast of Plane Island, to ensure that the species is dominant comparing to other limpet species along the sampling area; and focus on the abundance of this endangered species with the importance of Plane Island as a potential refuge; and to quantify the physical factors and levels pressure that may be leading to differences in limpet assemblage.
Material and Methods
Study area
Plane Island (Paloma Island) is located in the Eastern Alboran Sea (Western Mediterranean Basin), 7km offshore of Andalusia Bay (Oran Coast: Algeria) at 35º46.281’N and 0º54.115’W, between Cape Falcon and Cape Lindless. Plane Island is the second largest island of Oran after Habibas Islands in Oran. Its diameter is between 200 and 300m (Gueydon, 2005) and characterized by an amazing landscape and seascape as well as important biodiversity richness typical to Mediterranean islands. It shelters many endangered species such as the noble pen shell Pinna nobilis, the grouper Epinephelus marginatus and an important population of ferruginous limpets Patella ferruginea (Espinosa, 2009; Kallouche, 2018). To conserve this biodiversity, the island has been declared a protected marine area (MPA) since September 2018 (CCWO, 2018).
Methodology
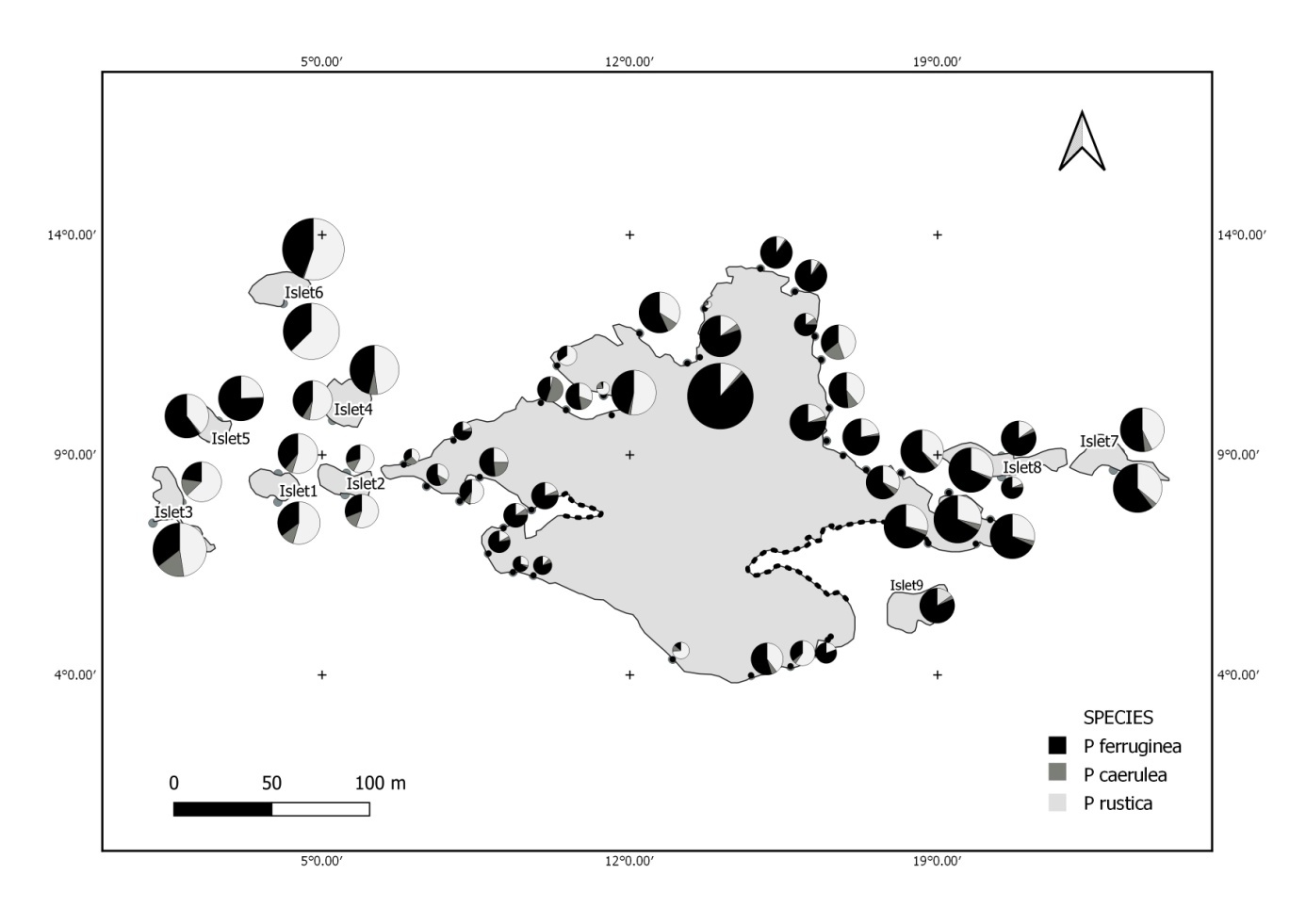
The following methodology is used to help us to know the real status of the ferrugineous limpet compared to other species limpet in all parts of our MPA. For this reason, the studied area has been divided into several zones: 8 in the main island, and 9 islets (Figure 1). Each one of this 17 zones has been subdivided in several intertidal transects. The islets were grouped according to their location into 4 clusters: Islets West (1, 2 and 3), North West (4, 5 and 6), East (7 and 8) and South West (9).
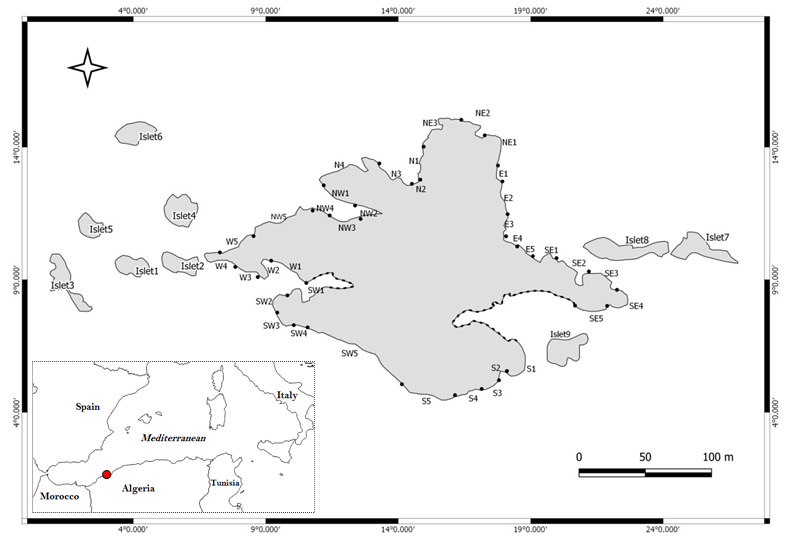
Sampling
Sampling was done from September 2018 to March 2019. The sampling was performed according to a non-destructive method: after identification, all founded limpets in each transect was recorded in a submersible tablet without being moved (Espinosa et al., 2005; Espinosa, 2009; Guallart et al., 2013c; Kallouche et al., 2014a; Guallart and Templado, 2016). All transects were recorded by their GPS points.
Data analysis
The significant results were performed by “Statistica 10” for abundance Index to differentiate limpet species population and distribution around our insular zone.
Mapping
The collected information about limpet abundance and the transect localisation were prepared to complete the data in QGIS software.
The primary GPS data and secondary data were organized before exporting to mapping with a suitable Excel format. The exported data was processed in QGIS software in order to change shape file data.
Results
Total limpet abundance
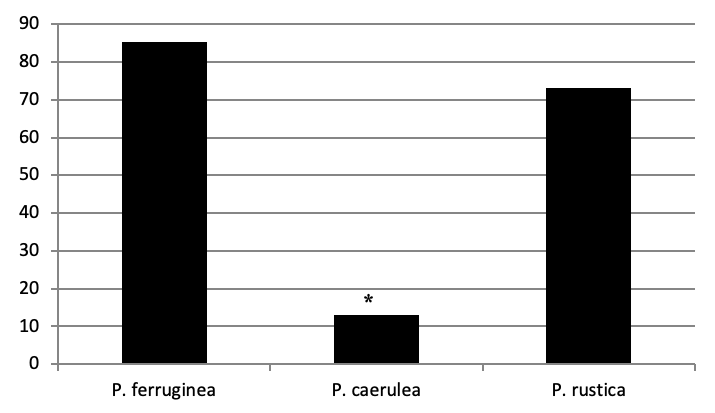
Three limpet species belonging to the Patella family were identified in Figure 3: the ferruginous limpet Patella ferruginea, the ponctuate limpet Patella rustica, and the blue Mediterranean limpet Patella caerulea. In addition, the false limpet Siphonaria pectinata are observed in Paloma Island. This last species is included in the Opistobranchia Order and exluded in our inventory.

Density and abundance of species
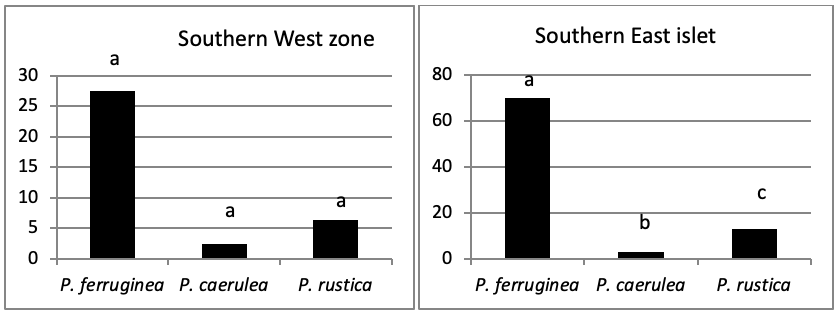
Of all individuals recorded on Plane Island, the ferruginous limpet is the more abundant species with 3993 individuals with a density of 1.78 ind/m (Figure 4). In the second position, P. rustica is represented by 1861 individuals with a density of 0.82 ind/m. In the last position, only 383 individuals of P. caerulea were founded with 0.17 ind/m.
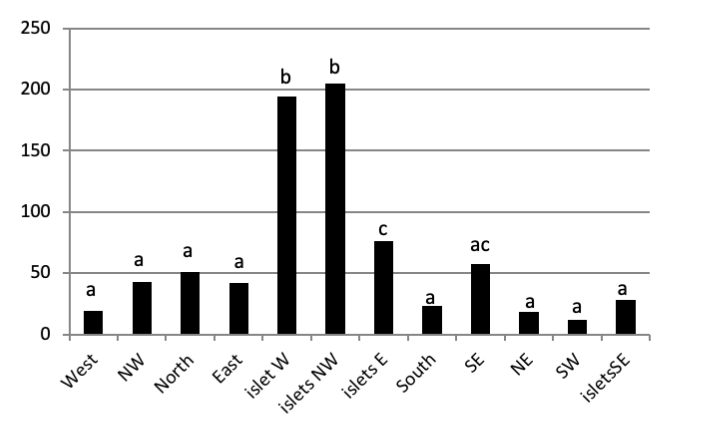
The total abundance, represented in Figure 2, is significantly very high on the West and North West islets, followed by the East zone and the South East islets with a lower abundance than the previous zones but still significantly higher than the remaining zones.
Concerning the main island, there is an absence of a significant result (P>0.05) in the distribution of limpet species between West, North West, East, South, North East, and South West (Figure 5).
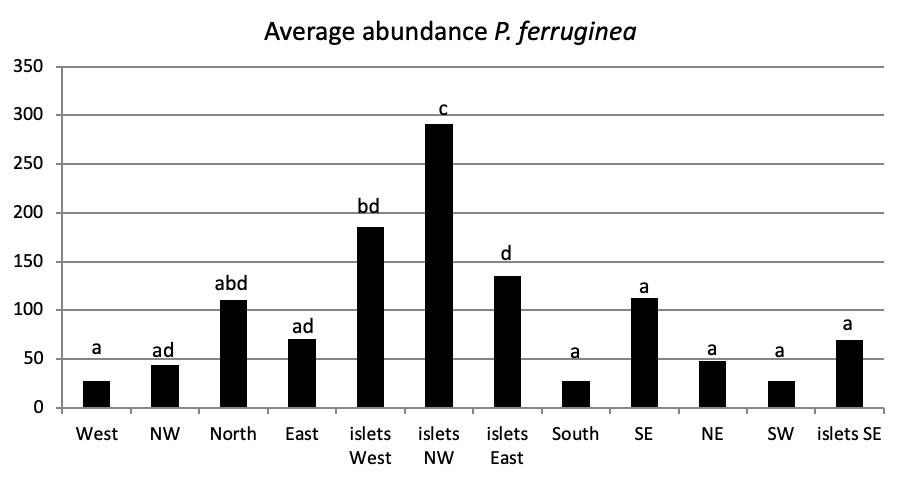
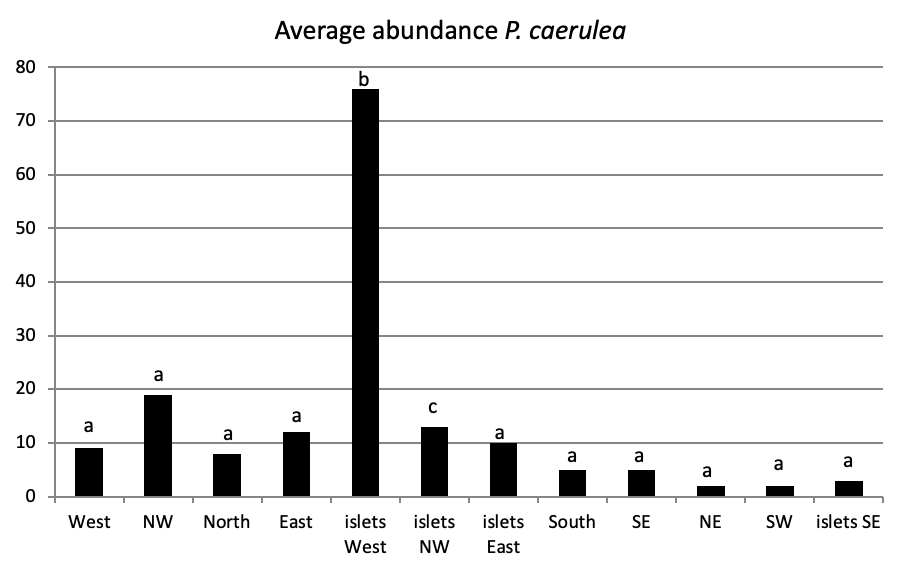
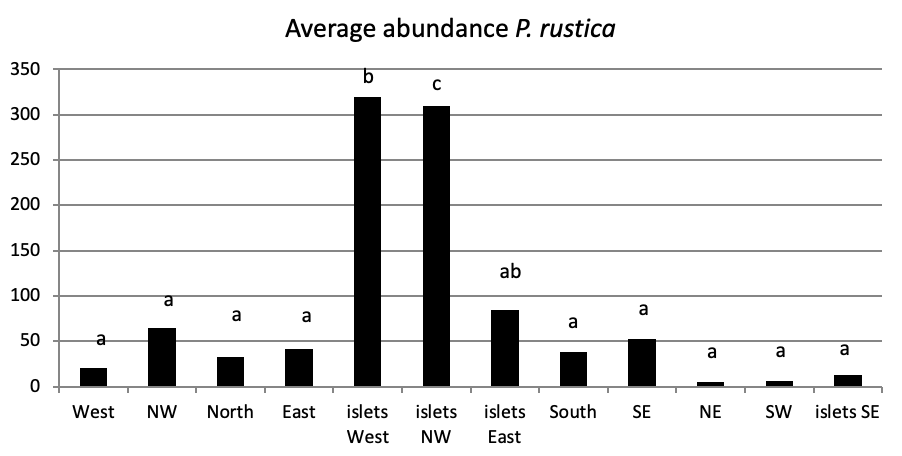
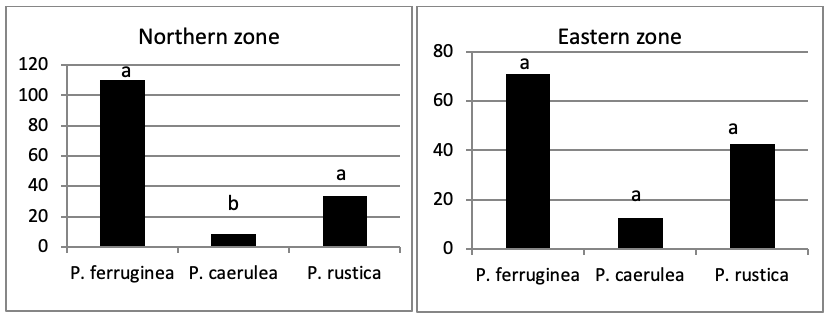
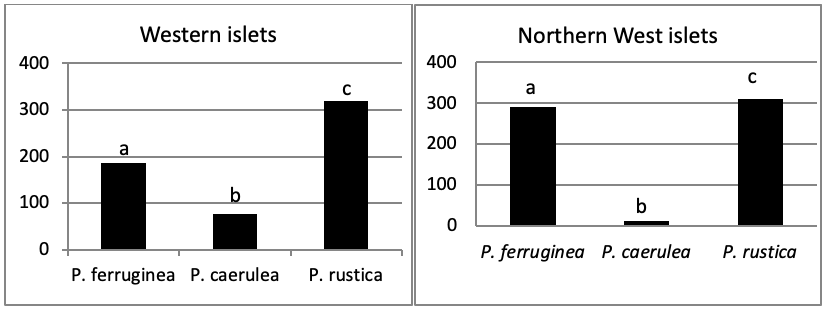
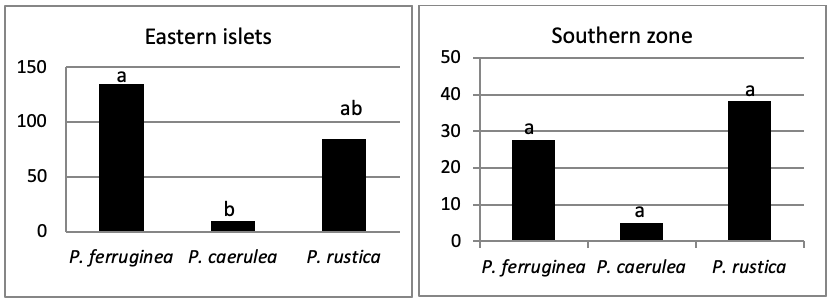
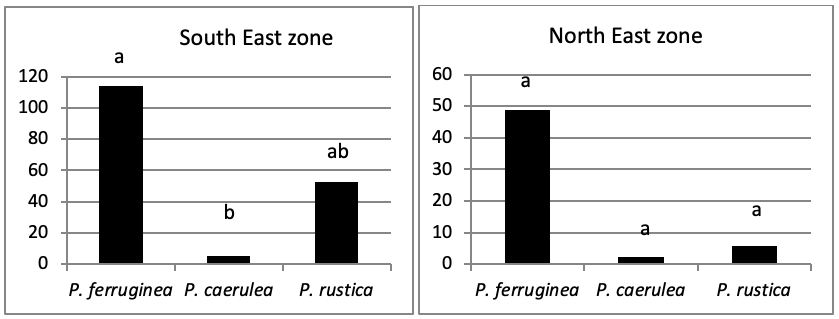
When comparing the abundance of all limpet species in relation to their location (Figure 5, the most significant abundance area for Patella ferruginea is found on islets and in the North, East and South-East zones of the main island. One notes also that the islet 6 is the most densest.
The mapping (Figure 6) shows the dominance of Patella ferruginea in the East zones. On the other hand, the Western zone is characterized by the dominance of P. rustica.
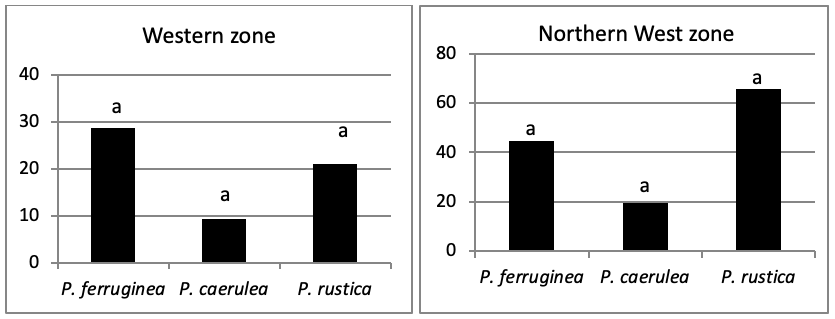
When areas are examined independently in Figure 7, the more significant abundance results were observed in the North of main island and islets (Duncan test, P<0,05). However, the East zone is the least populated area.
Principal Component Analysis (PCA)
The abundance of the three species of limpet recorded in the 17 areas of the insular zone throughout the study period was included in a multivariate analysis. This is in order to characterize and detect a correlation between the different zones in relation to the distribution of these Patellidae.
The PCA's results identify two main components includng PC1 = 78.98% and PC2 = 21.02% (Figure 8). PC1 is positively correlated with West islet, North West, North West, North West islets, North East, South, South East and North East. PC2 is positively correlated with all areas except East islets, South, South East and North East.
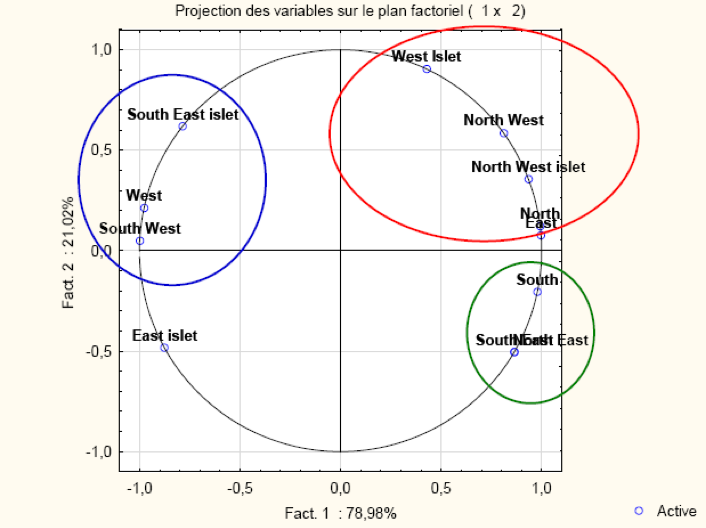
This analysis indicates significant correlations with factors ranging from 0.88 to 1 and reveals three groups of zones (Table 1). The first includes the West islets, North West, North West islets and North East areas. The second composed of the South, South East and North East zones. The last one is the South East, West and South West. The analysis clearly showed similarities in the density of the three limpet species in each group.
| West | North West | North | East | West Islets | North West islets | East islets | South | South East | North East | South West | South East islets | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| West | 1,00 | -0,67 | -0,94 | -0,96 | -0,23 | -0,84 | 0,75 | -1,00 | -0,95 | -0,95 | 0,99 | 0,90 |
| North West | 1,00 | 0,88 | 0,85 | 0,88 | 0,97 | -0,99 | 0,67 | 0,40 | 0,40 | -0,78 | -0,27 | |
| North | 1,00 | 1,00 | 0,54 | 0,97 | -0,93 | 0,95 | 0,80 | 0,80 | -0,99 | -0,70 | ||
| East | 1,00 | 0,50 | 0,96 | -0,91 | 0,96 | 0,82 | 0,82 | -0,99 | -0,74 | |||
| West Islets | 1,00 | 0,72 | -0,81 | 0,23 | -0,09 | -0,09 | -0,38 | 0,22 | ||||
| North West islets | 1,00 | -0,99 | 0,84 | 0,63 | 0,63 | -0,92 | -0,51 | |||||
| East islets | 1,00 | -0,76 | -0,51 | -0,51 | 0,85 | 0,39 | ||||||
| South | 1,00 | 0,95 | 0,95 | -0,99 | -0,90 | |||||||
| South East | 1,00 | 1,00 | -0,89 | -0,99 | ||||||||
| North East | 1,00 | -0,89 | -0,99 | |||||||||
| South West | 1,00 | 0,82 | ||||||||||
| South East islets | 1,00 |
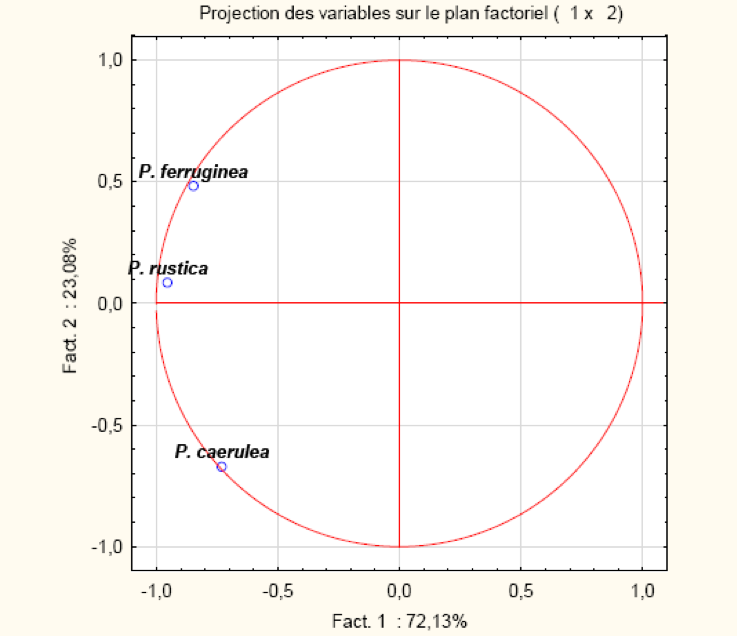
The overall PCA, representing the position of the three types of limpet, clearly separates P. ferruginea and P. rustica (R=0.79) from P. caerulea, indicating an abundance of the two first species relative to the third (Table 2; Figure 9).
| P. ferruginea | P. caerulea | P. rustica | |
|---|---|---|---|
| P. ferruginea | 1,00 | 0,32 | 0,79 |
| P. caerulea | 0,32 | 1,00 | 0,61 |
| P. rustica | 0,79 | 0,61 | 1,00 |
Discussion
The abundance recorded in the main areas of the island is much lower than that of the islets. However, we note in this study that Patella ferruginea is very abundant compared to other Patellidae.
The ferruginous limpet Patella ferruginea is highly endangered in the Western Mediterranean Basin where it is endemic (Boudouresque et al., 1996). In the past, it was very abundant, however nowadays it is in a great decline and it is confined to island areas of the North African coasts (Laborel-Deguen and Laborel, 1991; Espinosa and Bazairi, 2009), including Algeria which undoubtedly shelters the most important populations (Espinosa, 2009; Kallouche et al., 2014a; Kallouche et al., 2018), which ranks it among the highest density populations with Chafarinas Island (Spain) 4.82 ind/m (Guallart and Templado, 2016), Zembra (Tunisia) 2.65 ind/m (Espinosa et al., 2013; Zerrouk et al., 2016), Melilla 2.8 ind/m (Guallart et al., 2013c), and Ceuta 2.26 ind/m (Rivera-Ingraham et al., 2011). Following our sampling campaigns, it appears that the average population density of Patella ferruginea is 1.78 ind/m. On the other hand, the density of Patella caerulea — which is the most common species in the Mediterranean (Christiaens, 1973; Cretella et al., 1994; Guerra-Garcia et al., 2004) — reaches 125 ind/m in the Strait of Gibraltar area (Frenkiel, 1975; Templado, 1997). This limpet is the least abundant in our island with 0.7 ind/m. This result is due to the high currentology and hydrodynamic force exerted on the island. Indeed, Patella caerulea prefers the least turbulent coasts (Christiaens, 1973; Cretella et al., 1994; Guerra-Garcia et al., 2004). On Bousfer coast, on the opposite mainland from Plane Island (7km), P. caerulea is the most dominant limpet with 12.5 ind/m (Kallouche, 2018).
Also, we note the presence of Patella rustica on the study of Kallouche et al. (2014a), which indicates that its average density on the dike of the port of Oran is between 70 and 100 ind./m2. P. rustica is the species that predominates along the entire coast of Oran (Kallouche, 2018), while in our insular area, the density tends towards P. ferruginea despite the non-significant difference.
Understanding both the geographic range and the habitat use of a species is fundamental to characterizing its ecology; enabling predictions regarding how it will react to environmental changes (Gaston and Fuller, 2009).
The difference in Patellidae abundance, compared to the areas of Plane Island, is significant (P<0.05). It can be suggested that population density is influenced by several factors. This difference may be abiotic due to environmental conditions, particularly wave exposure and desiccation (Rilov et al., 2001; Kallouche et al., 2014; Kallouche, 2018). These differences seem to confer physical benefits for the survival of individuals in their ecological niches (Boulajfene et al., 2013). This may explain the high population densities of Patellidae in the areas and islets on the extreme East and West sides.
In addition, activities of anthropogenic origin leave significant nagtive effects on the limpets’ population (Dauvin, 2002). It is observed that the density of Patellidae is higher in the islets. This may be due to a lack of contact by fishermen who harm the limpets, often using them as bait, or dumping bleach on the intertidal rocky shores to obtain worms that will also be used as bait.
Indeed, areas with low limpet densities are pedestrian areas with frequent trampling by tourists and by fishermen (Helmuth et al., 2011). These areas are as follows: West, South West and North West. There is also a low abundance of limpets in the landing and the North-East zone known as the swimming pool, which is due to a lack of currentology. Indeed, hydrodynamics is the main source of oxygenation of Intertidal organisms (Rais et al., 2002).
The areas where the abundance of limpets (particularly the ferruginous limpet) is important are the 4 different islet groups NW/W/E/SE, since they constitute a favourable habitat for the survival of the different limpet species. It should be noted that these islets are relatively far from any pressure from fishermen and tourists. These islets are difficult for fishermen and tourists to access because of the distance from the main island. Moreover, these places constitute the first barriers against hydrodynamism and are therefore more oxygenated by wave action. Luque et al. (2018) hypothesized that physical factors such as wave exposure, acclivity, texture, microbial biofilm, rather than the mineralogy of the rocks, affect the distribution of the ferruginous limpet.
The Atlantic waters penetrate into the Mediterranean as surface waters, while denser than Mediterranean waters. This water is dispersed and collected in Algeria and then partially distributed towards the Provençal Basin and Catalan — forming the North-Balearic front current — where part of it will progress through the Sardinian Channel in its southern part, the North-Tunisia vein (Pierini and Rubino, 2001; Salas et al., 2002). It then joins the West Corsican vein so that the flow of Atlantic water reorganizes once again into the Western Basin. This gyre continues along the slope in Liguria, Provençal and Catalan, Algerian and enters Alboran where it closes the circle (Benzohra and Millot, 2005; Millot, 1999). This current direction is related with the dominance of Patella ferruginea in the East zones, and P. rustica in the Western zone. The post-larval recruitment is related by physical abiotic factors: the direction of marine current from the West (Atlantic origin) disperses P. ferruginea larvae from the West to the East, and these larvae are recruted in the East of the island. In addition, this same current bring larvae of P. rustica from Atlantic and these later are recruited in the Western of the island. The recruitment in the Eastern part of the island is supplied with larvae of P. ferruginea from the East currents (Algerian coasts) and the Western currents (larvae from the East side of the island). In addition, each zone is able to recruit post-larvae from itself.
It is evident that Plane Island constitutes a hotspot for P. ferruginea, which is a refuge for this endangered species on the one hand, and potentially disperse and drive larvae of P. ferruginea to continent coast on the other. As indicated in Ferranti et al. (2019) in Lugurian Sea, to be considered in order to understand the distribution of these species refers to the protection level of the island and the abbility of the continental zones to receive these larvae (current), ensure its recruitment (substratum quality), and especially guarantee its survival against human forces. Hence, the effectiveness of protected areas in guarding against the decline of ferruginous limpet population, by producing and dispersing larvae to ensure the continuous presence of continental specimens.
Intertidal environments are facing a global crisis as climate changes are causing sea-level rise. In relation to our hotspot island, with a 50cm rise in sea level, the South West part (between SW1 and SW2) would be submerged and the water would penetrate several tens of meters inside the island, which would significantly reduce the land surface of the island, with a direct consequence of reducing ferruginous limpet habitats. The ferruginous limpet created space for other plants and animals to move in, and we saw much more diversity and variety in these ecosystems. Variety is desirable because it helps protect the ecosystem when a stressor like heat is added. When limpets were part of the community, the effects of warming were less harsh (Kordas et al., 2017). Synergistically, other human-induced impacts (e.g., sewage, habitat loss, shell collection) caused increased natural vulnerability of intertidal insular ecosystems. However, the effect of these threats have long been neglected due, in part, to a limited knowledge of some aspects of intertidal ecology (Andrades et al., 2018).
In conclusion, distributional patterns will become established, and this study provides the first comprehensive baseline description of the physical factors affecting the status of the ferruginous limpet in limpet assemblage in Plane Island. The importance of our insular protected area is (i) that it constitues a refuge for P. ferruginea, and (ii) dispersal larvae ability and recruitment in other places far away from the island. In order to know the variability between the different limpet settlement, it must be necessary to extend this study in other coastal and insular zones to understand the mechanisms of the other factors that affect intertidal biodiversity, and last but not least, document future climate changes and sea level rise.
Acknowledgments
The authors would like to thank Chakouri Amine and Rouba Amine of the Marine Ecologic Association BARBAROUS for the logistic helping, and all fishermen that contributed in this study. Our thanks also to Ferhat Camélia for the English revision. This research study is supported and funded by Directorate General for Scientific Research and Technological Development (DGRSDT).
References
- Andrades, R., Amorim, Reis-Filho, J., Macieira, R.M., Giarrizzo, T., Joyeux, J.C., 2018. Endemic fish species structuring oceanic intertidal reef assemblages. Scientific Reports, 8, Article number: 10791.
- Aparici-Seguer, V., Guallart-Furia, J., Vicent-Rubert, J.J., 1995. Patella ferruginea population in Chafarinas Islands (Alboràn Sea, Western Mediterranean). In: Abstracts. Twelfth International Malacological Congress: 119-121 (A. Guerra, E. Rolen, and F. Rocja, Eds.). Instituto de Investigaciones Marinas (CSIC), Vigo.
- Beldi, H., Boumaza, F.Z., Draredja, B., Soltani, N., 2012. Biodiversité des Patellidae (Gastropoda, Prosobranchia) du golfe d’Annaba (Algérie nord-est), Bull. Soc. zool., 2012, 137(1-4): 121-132.
- Benzohra, M., Millot, C., 1995. Characteristics and circulation of the
surface and intermediate water masses of Algeria. Deep Sea Research, 42 (10): 1803-1830. - Biagi V., Poli D., 1986. Considerazioni su una populazione di Patella ferruginea Gmelin, 1791 per le acque del promontorio di Piombino. Bollettino Malacologico, 22: 171-174.
- Boudouresque, C.F., Avon, M., Gravez, V., 1991. Les espèces marines à protéger en Méditerranée, Ed. GIS Posidonie publication, France, 91-132.
- Boudouresque, C.F., Bianchi, C.N., 2013. Une idée neuve: la protection des espèces marines. In: GIS Posidonie: plus de 30 ans au service de la protection et de la gestion du milieu marin. Le Diréach L., Boudouresque C.F. (éds.), GIS Posidonie publ., Marseille: 85-91.
- Boudouresque, C.F., 2005. Excursion au Cap-Croisette (Marseille): le milieu marin. 12º édition. GIS Posidonie publishers, Marseilles, Fr.: 1-48.
- Boudouresque, C.F., Laborel-Deguen, F., 1986. Patella ferruginea, In: Boudouresque, C.F., Harmelin, J.G., Jeudy De Grissac, A. (Eds.), Le benthos marin d l’Ile de Zembra (Parc National, Tunisie), GIS Posidonie Publishers, Marseille: 105-110.
- Boudouresque, C. F., Beaubrun, P. C., Relini, G., Templado, J., Van Klaveren, M. C., Van Klaveren, P., Walmsley, J. G., Zotier, R., 1996. Critères de sélection et Liste révisée d’espèces en danger et menacées (marines et saumâtres) en Méditerranée, Programme des Nations Unies pour l’environnement (Rac/Spa Tunis), GIS Posidonie Publishers, Marseille, 73 pp.
- Boulajfene, W., Boukhicha, J., Ben Hassine, O.K., Tlig-Zouari, S., 2013. Distribution and demographic structure of Stramonita haemastoma (Linnaeus, 1767) populations on the rocky shores of the gulf of Tunis. Rapp. Comm. int. Mer Médit., 40, 659.
- Boumaza, S., Semroud, R., 2001. Inventaire de la population de Patella ferruginea Gmelin, 1791 des Iles Habibas (Ouest Algérien), Rapport du Congres de la Commission Internationale pour l’Exploration Scientifique de la Mer Méditerranée, 36: 361.
- Bouzaza, Z., Mezali, K., 2018. Evolution and Pattern of connectivity in some species of the genus Patella from the Algerian coast. Advances in Bioresearch, 9 (5): 46-53
- Branch, G.M., 1981. The biology of limpets: physical factors, energy flow and ecological interactions, Oceanogr. Mar. Ann. Rev., (19):235-380.
- CCWO, 2018. Classification commission of the wilaya of Oran, Wali decision Nº4961 of 17/09/2018.
- CMA-JA, 2014. Consejería de Medio Ambiente, Junta de Andalucía: Informe Final de Resultados 2014. Programa de Gestión Sostenible del Medio Marino Andaluz. Consejeríade Medio Ambiente y Ordenación del Territorio, Junta de Andalucía, p.126.
- Christiaens, J. 1973. Révision du genre Patella (Mollusca, Gastropoda), Bull. Mus. Natl. Hist. Nat., 3, 182: 1305-1392.
- Cretella, M., Scillitani, G., Toscano, F., Turella, P., Picariello, O., Cataudo, A., 1994. Relationships between Patella ferruginea Gmelin, 1791 and the other Tyrrhenian species of Patella (Gastropoda: Patellidae), Journal of Molluscan Studies, 60: 9-17.
- Curini-Galletti, M., 1979. Ritrovamento di una Patella ferruginea, Notiziario CISMA, 1: 53-54.
- Espinosa F., FA D.A., Ocaña T.M.J., 2005. Estado de la especie amenazada Patella ferruginea Gmelin, 1791 (Gastropoda: Patellidae) en la bahía de Algeciras y Gibraltar, Status of the endangered limpet Patella ferruginea Gmelin, 1791 (Gastropoda: Patellidae) in the Algeciras bay and Gibraltar, Iberus, 23 (2): 1-8.
- Espinosa, F., Guerra-Garcia, J.M., FA, D., Garcia-Gomez, J.C., 2006. Aspect of reproduction and their implications for the conservation of the endangered limpet, Patella ferruginea. Invertebrate, Reproduction and Development., 49: 85-92. DOI: 10.1111/j.1439-0469.2005.00348.x
- Espinosa, F., Gonzalez, A. R., Maestre, M. J., FA, D., Guerra-Garcia, J. M., Garcia-Gomez, J.C., 2008. Responses of the endangered limpet Patella ferruginea to reintroduction under different environmental conditions: survival, growth rates and life-history, Italian Journal of Zoology, 75 (4): 371-384.
- Espinosa, F., 2009. Populational status of the endangered mollusc Patella ferruginea Gmelin, 1791 (Gastropoda, Patellidae) on Algerian islands (SW Mediterranean), Animal Biodiversity and Conservation, 32 (1):19-28.
- Espinosa, F., Rivera-Ingraham, G. A., Maestre, M., González, R. A., Bazairi, H., García-Gómez, J. C., 2013. Updated global distribution of the threatened marine limpet Patella ferruginea (Gastropoda: Patellidae): an example of biodiversity loss in the Mediterranean. Fauna & Flora International, Oryx, 48(2): 266-275. DOI: 10.1017/S0030605312000580.
- Ferranti, M., Monteggia, D., Asnaghi, V., Dagnino, A., Gaino, F., Moretto, P., Parodi, V., Tixi, L., Cappanera, V., Valerani, C., Bava, S., Chiantore, M., 2019. Distribution of the Mediterranean ribbed limpet Patella ferruginea Gmelin, 1791 along the Ligurian coast and implications for conservation actions. Mediterranean Marine Science, 0, 496-501. DOI: 10.12681/mms.20209.
- Frenkiel, L., 1975. Contribution à l'étude des cycles de reproduction des Patellidae en Algérie. Publ. Staz. zool. Napoli, 39: 153-189.
- Frenkiel, L., Mouëza, M., 1982. Ecologie des Patellidae dans différents biotopes de la côte Algerienne. Malacologia, 22 (1-2): 523-530.
- Gaston, K.J., Fuller, R.A., 2009. The sizes of species’ geographic ranges. Journal of Applied Ecology. 46:1-9.
- Ghisotti, F., Melone, G. C., 1970. Catalogo illustrato delle conchiglie, 3-4 suppl., 39.
- Grande, C., Templado, J., Zardoya, R., 2008. Evolution of gastropod mitochondrial genome arrangements, BMC Evolutionary Biology, 8: 61. DOI: 10.1186/1471-2148-8-61, 15p.
- Grandfils Accino, R., 1982. Contribucion al conocimiento de Patella ferruginea (Gmelin, 1789) (Spana), Iberus, 2:57-69.
- Guallart, J., Calvo, M., Cabezas, P., 2006. Biología reproductora de la lapa Patella ferruginea (Mollusca: Patellidae), especie catalogada “en peligro de extinción” (Reproductive biology of the limpet Patella ferruginea (Mollusca, Patellidae), species classified as “under risk of extinction”). Barcelona: Book of Abstracts XIV Simposio Ibérico de Estudios de Biología Marina, p. 61.
- Guallart, J., Calvo, M., Cabezas, P., 2010. Hermafroditismo en la lapa ferruginosa (Patella ferruginea) (Mollusca, Patellidae), especie catalogada «en peligro de extinción» [Hermaphroditism in the ferruginous limpet (Patella ferruginea) (Mollusca, Patellidae), species classified as «under risk of extinction», Alicante: Book of Abstracts XVI Simposio Ibérico de Estudios de Biología Marina, 150 p.
- Guallart, J., Acevedo, I., Templado, J., 2011. Año excepcional para la lapa ferrugínea en Chafarinas (an exceptional year for the ferruginous limpet in Chafarinas), Quercus, 307:60-61.
- Guallart, J., Templado, J., 2012. Patella ferruginea. En: VV.AA., Bases ecológicas preliminares para la conservación de las especies de interés comunitario en España: Invertebrados. Ministerio de Agricultura, Alimentación y Medio Ambiente, Madrid, 86 pp.
- Guallart, J., Acevedo, I., Calvo, M., 2012. Reclutamiento de la lapa amenazada Patella ferruginea (Mollusca, Patellidae) en las Islas Chafarinas (Mediterráneo SW) [Recruitment of the endangered limpet Patella ferruginea (Mollusca, Patellidae) in Chafarinas Islands (SW Mediterranean)]. Cádiz: Book of Abstracts, III Simposio Internacional de Ciencias del Mar. p. 15.
- Guallart, J., Calvo, M., Acevedo, I., Templado, J., 2013a. Two-way sex change in the endangered limpet Patella ferruginea (Mollusca, Gastropoda), Invertebrate Reproduction & Development, only online: 10.1080/07924259.2012.754794.
- Guallart, J., Acevedo, I., Calvo, M., Machordom, A. 2013b. Protocolo no letal para la obtención de muestras de tejido (para estudios genéticos) en la lapa amenazada Patella ferruginea (Gastropoda, Patellidae), Non-lethal protocol for tissue-sampling (for genetic studies) in the endangered limpet Patella ferruginea (Gastropoda, Patellidae), Iberus, 31 (2): 171-174.
- Guallart, J., Luque, A. A., Acevedo, I., Calvo, M., 2013. Distribución y censo actualizado de la lapa ferrugínea (Patella ferruginea Gmelin (1791) en el litoral de Melilla (Mediterráneo suroccidental) Distribution and updated census of the ferrugineous limpet (Patella ferruginea Gmelin, 1791) in the littoral of Melilla (SW Mediterranean), Iberus, 31 (1): 21-51, 2013.
- Guallart, J., Templado, J., 2016. Distribution, abundance and habitat selection of Patella ferruginea in Chafarinas Islands (Southwestern Mediterranean Sea), Iberus, 34: 127-162.
- Guerra-Garcia, J.M., Corzo, J., Espinosa, F., Garcia-Gomez, J.C., 2004. Assessing habitat use of the endangered marine mollusc Patella ferruginea (Gastropoda, Patellidae) in Northern Africa: preliminary results and implications for conservation. Biological Conservation, 116 (3): 319-326.
- Gueydon, J., 2005. Les îles du littoral algérien, Revue L'algérianiste, juin 2005, Ed Saint Georges d'Orques, France, 110:54-55.
- Helmuth, B., Yamane, L., Lalwani, S., Matzelle, A., Tockstein, A., Gao, N., 2011. Hidden signals of climate change in intertidal ecosystems: What (not) to expect when you are expecting, Journal of Experimental Marine Biology and Ecology, 400: 191-199.
- Holmes, S.P., Cherrill, A., Davies, M.S., 2002. The surface characteristics of pedal mucus: a potential aid to the settlement of marine organisms, The Northumbrian Water Ecology Centre, The Science Complex, Sunderland, Tyne & Wear, SR1 3SD, UK, Journal of the Marine Biological Association of the UK, 82: 131-139.
- JORAPD, 2006. Official Journal of Algerian democratic and popular republic, Nº 75, 45th year, 22 November, 2006.
- Kallouche, M.M., Bouras, D., Ghalek, M., Abdelghani, F., 2011. Aspect et répartition de la patelle commune méditerranéenne (Patella caerulea) de la zone côtière oranaise (littoral algérien occidental), Proceeding Conférence Méditerranéenne Côtière et Maritime. EDITION 2, TANGER, MAROC. Disponible en ligne - http://www.paralia.fr: 350-360. DOI: 10.5150/cmcm.2011.074.
- Kallouche, M. M., Bouras, D., Hussein, K. B., 2014a. Faunal composition, distribution and richness of the Oran’s intertidal coastal zone (Mediterranean Sea, Algeria), Journal of Biodiversity and Environmental Sciences, 5 (4): 122-132.
- Kallouche, M. M., Bouras, D., Hussein, K. B., 2014b. The biotic and abiotic factors affecting the biodiversity on intertidal zone of the Mediterranean Sea: Algerian west coast case, Proceeding of bel 03, 26-28 November 2013, Oran, Algeria: 225-232.
- Kallouche, M. M., Acevedo, I., Ghalek, M., Bouras, D., Machordom, A., 2018. Filling the limpet gap: molecular characterization of the genus Patella (Patellidae, Gastropoda) in the algerian coasts of Oran, Acta Zoologica Academiae Scientiarum Hungaricae, 64(2): 161-184. DOI: 10.17109/AZH.64.2.161.2018.
- Kallouche, M. M., 2018. Dynamique spatiotemporelle de la structure écobiologique des patelles de la zone côtière oranaise, Thesis of Doctorat, Univ. Oran 1, 240p.
- Kordas, R.L., Donohue, I., Harley, D.G.C., 2017. Herbivory enables marine communities to resist warming . Sci. Adv. 3 (10): e1701349. DOI: 10.1126/sciadv.1701349
- Laborel-Deguen, F., 1985, Biologie et répartition de Patella ferruginea, Tavaux Scientifique du Parc Naturel régional et Réserve Naturelle d Corse, France, 2: 41-48.
- Laborel-Deguen, F., Laborel, J., 1990. Nouvelles données sur la patelle géante Patella ferruginea Gmelin en Méditerranée. I. Statut, répartition et étude des populations II Ecologie, Biologie, Reproduction, Haliotis, 10: 41-62.
- Laborel-Deguen, F., Laborel, J., 1991a. Statut de Patella ferruginea Gmelin en Méditerranée. In: Boudouresque C.F., Avon M., Gravez V. (Eds.), Les espèces marines à protéger en Méditerranée, GIS Posidonie Publishers, Marseille, 91-103.
- Laborel-Deguen, F., Laborel, J., 1991b. Nouvelles observations sur la population de Patella ferruginea Gmelin de corse, In: Boudouresque C.F., Avon M., Gravez V. (Eds.), Les espèces marines à protéger en Méditerranée, GIS Posidonie Publishers, Marseille, 119-128.
- Laborel-Deguen, F., Laborel, J., Morhange, C., 1993. Appauvrissement des populations de la patelle géante Patella ferruginea Gmelin, 1791 (Mollusca, Gasteropoda, Prosobranchiata) des côtes de la Réserve marine de Scandola (Corse du Sud) et du Cap Corse (Haute Corse). Trav. sci. Parc nat. rég. Rés. nat. Corse, 41: 25-32.
- Luque, A.A., Guallart, J., Templado, J., Pola, M., 2018. Recompilacion y analisis de la informacion cientifica disponible sobre Patella ferruginea. Sociedad Española de Malacologia, Madrid, 250 pp.
- Doukara Larbi, K., 2019. Density and ecological aspect of endangered limpet Patella ferruginea in the western Algerian coast: Implications for the Conservation. Egyptian Journal of Aquatic Biology & Fisheries. 23(1): 65-76.
- Laurent, P., 1939, Une collection de coquilles recueillies à Gouraya, département d’Alger, pendant l’hiver 1936-1937, J. Conchiol., 83 (4): 318-326.
- Machordom, A., Ramirez-Escobar, U., Acevedo, I., Garcia-Jimenez, R., Cabezas P., Calvo M., Toledo C. Bloor P., 2010. Isolation and characterization of polymorphic microsatellite markers for the endangered ferruginous limpet Patella ferruginea (Gastropoda, Patellidae), Conserv. Genet., 11: 1083-1086.
- Millot, C., 1999. Circulation in the Western Mediterranean sea. J. Mar. Systems, 20 (1-4): 423-442.
- Moreno, D., 1992. Presencia de Patella ferruginea Gmelin, 1791 en el Cabo de Gata (Almeria, SE España), Cuadernos Investigacion Biologica, 17: 71.
- Pallary, P., 1900. Coquilles marines du littoral du Département d'Oran, Journal de Conchyliologie, 48(3): 211-422.
- Parauellos, M., Nevado, J.C., Moreno, D., Giménez, A., Alesina, J.J., 2003. Conservational status and demographic characteristics of Patella ferruginea Gmelin, 1791 (Mollusca, Gastropoda) on the Alboran Island (Western Mediterranean), Animal Biodiversity and Conservation, 26 (2): 29-37.
- Porcheddu, A., Milella, I., 1991. Aperçu sur l’ecologie et sur la distribution de Patella ferruginea (L.) Gmelin, 1971 en mers italiennes. In: Boudouresque C.F., Avon M. and Gravez V., Ed. GIS Posidonie publ., 119-128.
- Rais, C., Tunesi, L., Agnesi, S., Di Nora, T., Manca Zeichen, M., M., Mo G., Molinari A., Elena Piccione M., Salvati E., Benhissoune S., Bazairi H., Nachite D., Sadki I., Benhamza A. H. Franzosini C., 2002. Projet régional pour le Développement d’Aires Protégées Marines et Côtières dans la région méditerranéenne (PROJET MedMPA), Rapport de la première mission de prospection de la partie marine du parc national d’Al Hoceima. Maroc, 105 p.
- Ramos, M. A., 1998. Implementing the Habitats Directive for mollusc species in Spain. Journal of Conchology, 2: 125- 132.
- Rilov, G., 2001. Ecological, Management, and Geographic Perspectives. The population biology of invasive species, 110: 282-283.
- Rivera-Ingraham, G. A., Espinosa, F., Garcia-Gomez, J.C., 2011. Environmentally mediated sex change in the endangered limpet Patella ferruginea (Gastropoda Patellidae), Journal of Molluscan Studies, 77: 226-231.
- Taibi, A, Oubaziz, B, Ghermaoui, M, Kaddour Hocine, A, Bendimerad, M.E., 2014. Etude de la biométrie de la Patelle géante Patella ferruginea à l’île de Rachgoun, Proceeding of bel 03, 26-28 novembre 2013, Oran, Algérie: 584-592.
- Templado, J. 1996. Patella ferruginea (Gmelin, 1791). In: M.A. Ramos (Ed.), Inventario de las especies de invertebrados no artropodos incluidas en los anejos de la Directiva 92/43/CCE del Consejo, pp. 1-15. Memoria final (ICONA, unpublished).
- Templado, J., 1997. La lapa ferruginea, Biologica, 6: 80-81.
- Templado, J., Calvo, M., Garvía, A., Luque, A. A., Maldonado, M., Moro, L., 2004: Guía de invertebrados y peces marinos protegidos por la legislación nacional e internacional. Organismo Autónomo de Parques Nacionales, Madrid. 214 p.
- Tlig-Zouari, S., Rabaoui, L., Fguiri, H., Diawara, M., Ben Hassine, O., 2010. Spatial diversity of rocky midlittoral macro-invertebrates associated with the endangered species Patella ferruginea (Mollusca: Gastropoda) of Tunisian coastline, Estuarine Coastal and Shelf Science, 87: 275-283.
- Zarrouk, A., Romdhane, M.S., Espinosa, F., 2016. Usefulness of marine protected areas as tools for preserving the highly endangered limpet, Patella ferruginea, in the Mediterranean Sea. Marine Biology Research, 12 (9): 917-931.
