Diversity and structural complexity of mangrove forest along Puerto Princesa Bay, Palawan Island, Philippines
Palawan State University-Marine Science Laboratory, Puerto Princesa City, Palawan, Philippines fddgalon@yahoo.com
College of Fisheries and Aquatic Sciences, Western Philippines University, Puerto Princesa Campus, Palawan, Philippines
Palawan State University-Marine Science Laboratory, Puerto Princesa City, Palawan, Philippines
Palawan State University-Marine Science Laboratory, Puerto Princesa City, Palawan, Philippines
Abstract
The paper describes the diversity and structural complexity of mangrove forest along Puerto Princesa Bay, Palawan Island, Philippines. Occurrence of 28 mangrove species and 11 floral associates were found, which identifies the entire bay as one of the most diverse mangrove forests in the country. Of the six coastal barangays surveyed, San Pedro had the highest diversity index, H′ = 0.912 while Sta Monica had the lowest, H′ = 0.349. Mangrove stands are structurally simple with two types of vegetation, fringe and riverine that further constitute five distinct mangrove zones named according to dominating species, Rhizophora-Sonneratia; Rhizophora-Sonneratia-Lumnitzera; Rhizophora-Lumnitzera-Xylocarpus; Rhizophora-Xylocarpus; and Rhizophora-Avicennia. Commonality among these zones is obvious as revealed in Bray-Curtis cluster analysis. Structural features differed across zones. Trees of larger dbh, 104.5 cm and higher species richness, a total of 15 species, were found in zone 1 while those that comprised the highest basal area, 438 m−2 ha−1 and density, 8100 ha−1 from zones 2 and 4, respectively. Zones 1 and 4 are fringing mangrove forests. Degrees of perturbations greatly depend on human access to mangrove areas. Garbage dumping, occasional cutting of trees, soil erosion, and encroachment of human settlers were identified as potential threats to mangrove forest along the bay.
Keywords
Puerto Princesa Bay, Mangroves, Species, Diversity, Structure
Introduction
Mangrove forest is one of the vital ecosystems in tropical countries. The Philippines alone is a home to at least 39 mangrove species (Sinfuego and Bout, 2014; Primavera et al., 2004) and similar to other regions, the various natural products and ecological services (Rönnbäck, 1999; Clough, 2013) of this resource are well recognized in the country, including its role in climate change mitigation (Donato et al., 2011; Sheeran, 2006).
Palawan has an extensive mangrove forest at 51,346 ha in 1998, representing 3.63% of the total land area of the province (PCSDS, 2004). Presidential proclamation 2152 of 1981 declared the entire island province as “Mangrove Swamp Forest Reserve” (http//www.puertoprincesa.ph, 2012). To date, nearly 31, 507 ha of mangrove forest in the island-province are highly protected under the International Union for Conservation of Nature (IUCN), and three species, Ceriops decandra, Aegiceras floridum and Lumnitzera littorea with threatened conservation status (Long and Giri, 2011) are widely distributed in some of its localities.
Mangrove ecosystems in nearly all municipalities of Palawan are well documented. Accordingly, Palawan has 23 “true mangrove” species. Species richness ranged from 8 to 17 per municipality of which Dumaran and Roxas in northern part of the island have the maximum number of species. Rhizophora apiculata, Sonneratia alba, and Bruqueira gymnorrhiza dominate the mangrove flora of the island (PCSDS, 2004, 1999).
Mangroves of Puerto Princesa constitute 11.7% (5995 ha) of Palawan’s mangrove cover. Major mangrove forests in the city have low volume stands due to deforestation rate of 10 has per year, which may be related to increasing number of fishponds, 530 units in 1998 from only 103 units in 1992 (PCSDS, 2004). In Puerto Princesa Bay alone, 150 ha mangrove area is covered by Fishpond Lease Agreement (FLA) (http//www.puertoprincesa.ph, 2012).
Available information on mangrove composition and structure along Puerto Princesa Bay are scarce. The latest available reference reported 18 true mangrove species from nine coastal barangays along the bay (PCSDS, 2006). Earlier attempts made to assess the status of mangroves in the area gave insufficient record on species composition, only 5–14 species, due to limited number of areas surveyed (Becira, 2005; Aliño et al., 2001). Nonetheless, mangrove species composition, community structure, growth, and recruitment in some portions of the bay were initially investigated.
This study was conducted as part of the Commission on Higher Education’s Research and Development Program for Marine Biodiversity along Bohol and Sulu Seas. After more than a decade, additional accounts on mangrove’s diversity and structural complexity from Puerto Princesa Bay are wanting.
Materials and methods
The study site
Puerto Princesa Bay, 9°40′N to 9°47′N and 118°40′E to 118°47′E, is a relatively shallow bay located in the mid-eastern coast of mainland Palawan, south of Puerto Princesa City. It covers 20 coastal barangays with a land area of 25,688 ha (www.puertoprincesacity.gov.ph, 2012). Mangrove survey was conducted in six coastal barangays along the bay namely; San Pedro, Tiniguiban, Sicsican, Irawan, Iwahig and Sta. Lucia (Fig. 1).
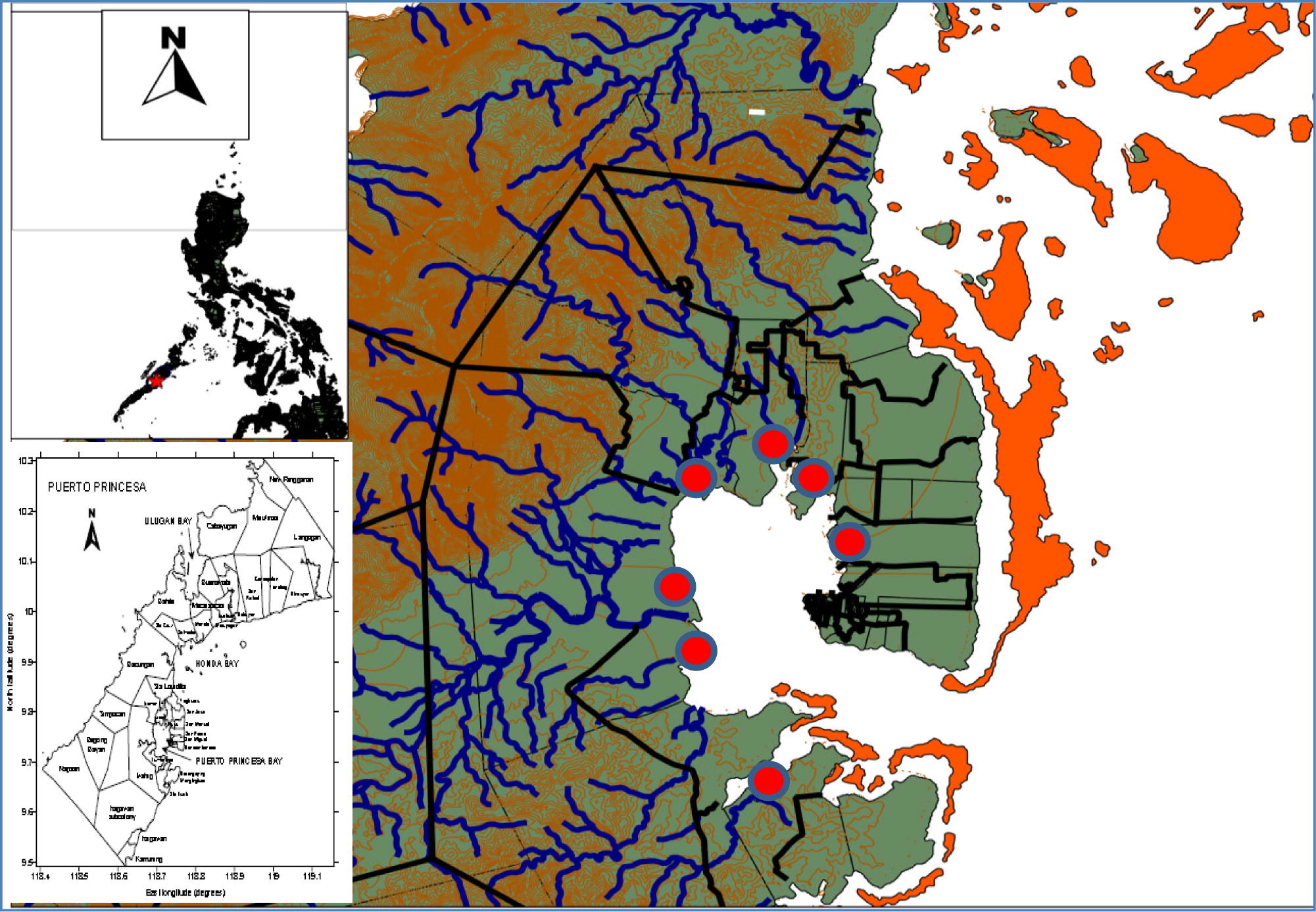
Sampling techniques
Mangrove vegetation assessment followed the standard protocol described by English et al. (1997) with slight modification. In each sampling station, at least two transects, 50–100 m, depending on the extent of mangrove cover, were laid perpendicular to the shore and/or riverbank. A 10 m × 10 m plot was established along the transect line at certain interval, 10–50 m, depending on the prevailing species zonation pattern.
Plant identification and data analyses
Individual plants found within the plot were identified following the nomenclature of Primavera and Sadaba (2012), Primavera et al. (2004), Feller and Sitnik (1996), and Tomlinson (1986). For each identified species, basic vegetation parameters such as the diameter at breast height (dbh), basal area, and density were measured. Data analyses utilized the Biodiversity Professional Software (McAleece et al., 1997) for species diversity indices (Shannon-Weiner) and multivariate (Bray-Curtis) tests. Direct observation on the surrounding environment of each plot was likewise done to record the existing potential threats to mangrove resource in the area.
Human-resource interaction
Human-related disturbances to mangrove forest were assessed using through actual observation by the researchers. Disturbances observed were verified using the Focused Group Discussion (FGD) and the Key Informant Interview (KII). Information generated were analyzed through descriptive statistics e.g. the frequency count and percentages.
Results
Species diversity
A total of 28 mangrove species and 11 floral associates were recorded from Puerto Princesa Bay. This represents 27 true mangrove species from 14 families and 15 genera (Table 1). Species richness differed across sampling sites of which barangay San Pedro had the highest diversity index (H′ = 0.912) followed by barangays Iwahig Station 4 and Purok Sandiwa, San Pedro with 0.768 and 0.760 H′ indices, respectively. Sta Monica had the lowest diversity index, H′ = 0.349 despite having a total of eight mangrove species (Table 2). Three IUCN threatened and near-threatened species, C. decandra, A. floridum and L. littorea were found in the study area.
| Family | Scientific name | Local name |
|---|---|---|
| True Mangroves | ||
| Acanthaceae | Acanthus ebracteatus Vahl. | Lagiwliw, ragoyroy |
| Avicenniaceae | Avicennia marina (Forsk.) Vierh | bungalon, api-api |
| Avicennia officinalis L. | bungalon, api-api | |
| Avicennia rumphiana Hall. f. | bungalon, api-api | |
| Bombacaceae | Camptostemon philippinensis (Vidal) Becc | gapas-gapas |
| Combretaceae | Lumnitzera littorea (Jack) Voigt. | libato, tabao |
| Lumnitzera racemosa Willd | libato, tabao | |
| Euphorbiaceae | Excoecaria agallocha L. | buta-buta |
| Meliaceae | Xylocarpus granatum Koen | tabigi |
| Xylocarpus moluccensis (Lam.) M. Roem | piagao | |
| Myrsinaceae | Aegiceras corniculatum (L.) Blanco | tinduk-tindukan |
| Aegiceras floridum Roem. and Schult | tinduk-tindukan | |
| Myrtaceae | Osbornia octodonta F. Muell. | tawalis |
| Arecaceae | Nypa fruitican (Thunb.) Wurmb. | nipa, sasa |
| Pteridaceae | Acrostichum aureum | palaypay, lagolo |
| Acrostichum speciosum | palaypay, lagolo | |
| Rhizophoraceae | Bruguiera cylindrical (L.) Blume | pototan, busain |
| Bruguiera gymnorrhiza (L.) Lam. | pototan, busain | |
| Bruguiera sexangula (Lour.) Poir. | pototan, busain | |
| Ceriops decandra (Gridd.) Ding Hou | malatangal | |
| Ceriops tagal (Perr.) C.B. Rob. | tangal | |
| Rhizophora apiculata Blume | bakaw lalaki | |
| Rhizophora mucronata Lam. | bakaw babae | |
| Rhizophora stylosa Griff. | bakaw bato | |
| Scyphiphora hydrophyllacea Gaertn. | nilad | |
| Rubiaceae | Sonneratia alba J. Smith | pagatpat |
| Sonneratiaceae | Sonneratia caseolaris (L.) Engl. | pedada |
| Sterculiaceae | Heritiera littoralis Dryand. Ex W. Ait. | dungon, dungon-late |
| Floral associates | ||
| Apocynaceae | Cerbera manghas Linn. | baribai, buta-buta |
| Asclepedeaceae | Hoya sp. | wax vine |
| Combretaceae | Terminalia catappa Linn. | talisay, kalisai |
| Euphorbiaceae | Glochidion littorale Blume | bagnang lalaw, sagasa |
| Fabaceae | Breynia vitis-idaea (Burm.f.) C.E.C..Fischer | matang-ulang, sungut-olang |
| Abrus precatorius Linn. | saga-saga, oyang-ya | |
| Albizia retusa Benth. | kasay, balunos | |
| Caesalpinia crista Linn. | bayag-kambing, dalugdug | |
| Caesalpinia sappan Linn. | sibukao, sappan | |
| Derris trifoliate Lour. | asim-asiman, sabuko | |
| Millettia pinnata (Linn.) Panigrahi | bani, balikbalik | |
| Flagellariaceae | Flagellaria indica Linn. | baling-uai,huak, uag |
| Malvaceae | Talipariti tiliaceum (Linn.) Fryxell | balibago, malabago |
| Rubiaceae | Morinda citrifolia Linn. | nino, lino, apatot |
| Zone | Sampling station (Barangay) | Shannon diversity index (H′) |
|---|---|---|
| 1 | Tiniguiban | 0.739 |
| Sandiwa, San Pedro | 0.760 | |
| Sta. Lucia (Station 1) | 0.676 | |
| 2 | Sta. Lucia (Station 4) | 0.627 |
| Iwahig (Station 4) | 0.768 | |
| Iwahig (Station 3) | 0.604 | |
| Iwahig (Station 2) | 0.749 | |
| 3 | Sta. Monica | 0.349 |
| Sicsican | 0.581 | |
| Iwahig (Station 1) | 0.567 | |
| Sta. Lucia (Station 2) | 0.470 | |
| 4 | Sta. Lucia (Station 3) | 0.515 |
| 5 | Irawan | 0.598 |
| San Pedro | 0.912 |
Vegetation types
Cluster analysis classified the 14 sampling sites into five distinct zones based on species composition and basal area (Fig. 2). Zone 1 includes three sampling sites, the Tiniguiban (TNG), Purok Sandiwa (SDW), and Sta. Lucia- Station 1 (STL1). The largest dbh recorded from this zone belonged to L. littorea (65.7 cm) but the highest basal areas were observed from S. alba at 41.53, 39.88, and 103 m2/ha in TNG, SDW, and STL1, respectively. Zone 2 includes the Sta. Lucia- Stn 4 (STL4), Iwahig- Stn 4 (IWH4), Iwahig- Stn 3 (IWH3), and Iwahig- Stn 2 (IWH2). Xylocarpus granatum had the largest dbh (69.62 cm) and highest basal area in IWH2 and IWH3 with 142 and 103.75 m2/ha, respectively while the L. littorea in STL4 with 100.22 m2/ha. Zone 3 comprises the mangroves of Sta. Monica (STM), Sicsican (SSC), Iwahig Stn 1 (IWH1) and Sta. Lucia Stns 2 and 3, (STL2 and STL3) with S. alba having the largest dbh and highest basal areas, 74.16 cm and 31.77 m2/ha, accordingly. Mangroves of Irawan (IRW) constitutes zone 4 with Xylocarpus mollucensis having the largest dbh (21.3 cm) and highest basal area (46.36 m2/ha). Transect 1 in this zone is a reforested area dominated by Rhizophora mucronata and Rhizophora stylosa with dbh ranging from 5.0 to 7.0 cm. Such dbh were comparably smaller than Rhizophora from natural stands with 12.8 cm dbh. Zone 5 is represented by mangrove vegetation of San Pedro with Avicennia marina as the biggest tree (37.4 cm dbh) and R. apiculata (23.05 m2/ha) and S. alba (21.94 m2/ha) having the relatively higher basal areas.
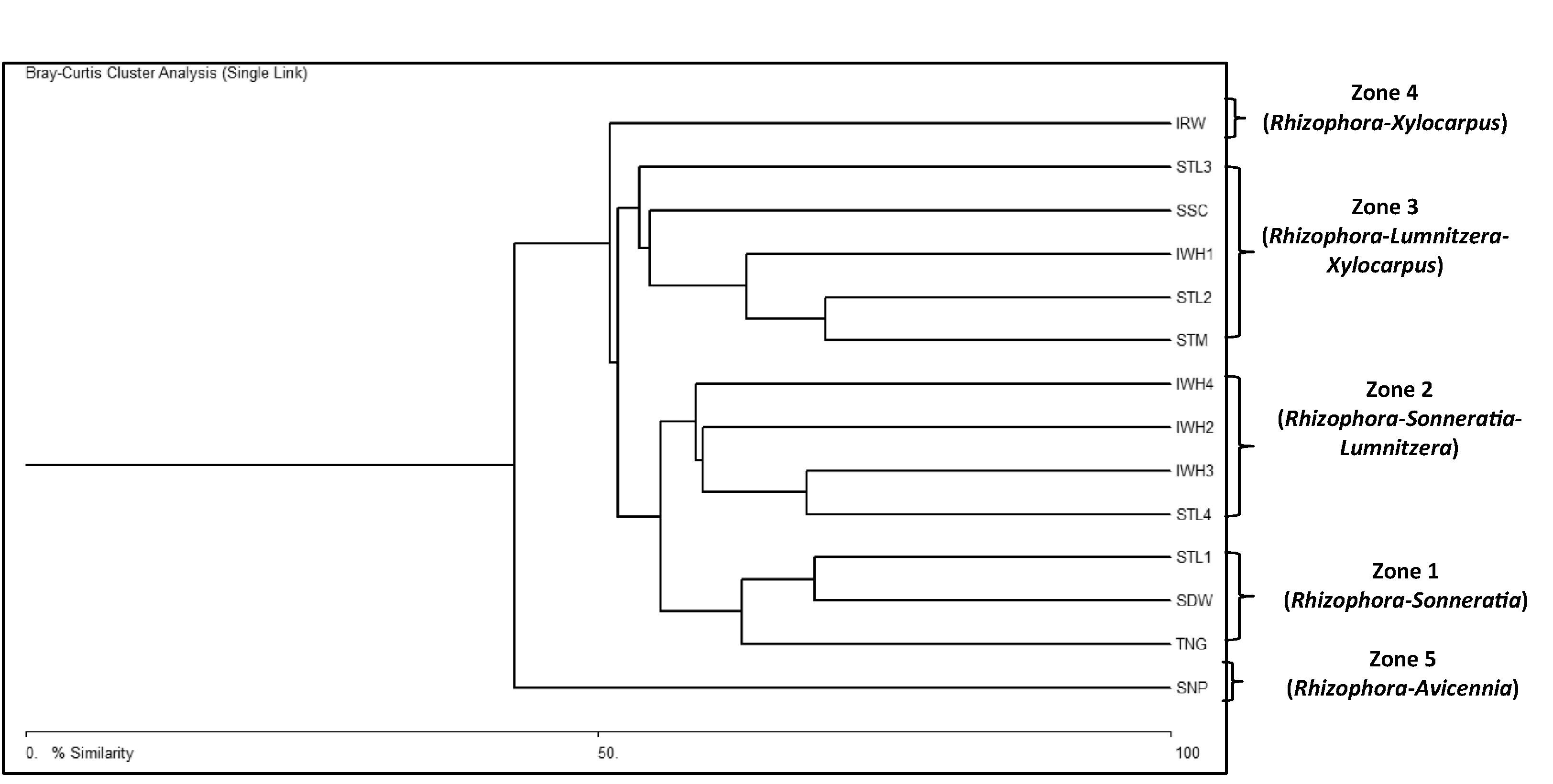
Physiographical classification based on location simply grouped these zones into two forest types, fringe and riverine. Riverine mangrove persists along the Iwahig and Sta Lucia river- estuaries, including the STL4, IWH2, IWH3, and IWH4 while fringing forest is formed toward the river mouth of Iwahig and Sta. Lucia such as the STL2 and STL3 and along the open coasts of Tiniguiban and Iwahig (Fig. 3).
Relative dominance further examined the differences among mangrove forests based on species importance in the overall community structure. These were derived from percent relative frequency, relative density, and relative basal area of mangrove species in the study sites (Table 3). Correspondingly, the existing five mangrove zones in Puerto Princesa Bay can be named according to their respective dominant species, Rhizophora-Sonneratia; Rhizophora-Sonneratia-Lumnitzera; Rhizophora-Lumnitzera-Xylocarpus; Rhizophora-Xylocarpus; and Rhizophora-Avicennia zones. Evident from the cluster diagram was the overlap between (zone 1 and 2) and among (zones 1–2 and 3; zones 1–2–3 and 4; zones 1–2–3–4 and 5) zones, implying some commonality that is a normal trend in the natural environment.
| Mangrove scientific name | Relative dominance (%) in five sampling zones | Total | Rank | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| Aegiceras corniculatum | 7.8 | 7.8 | 14 | ||||
| Aegiceras floridum | 16.1 | 16.1 | 12 | ||||
| Bruguiera sexangula | 12.3 | 10.2 | 22.5 | 10 | |||
| Ceriops decandra | 10.0 | 18.1 | 10.8 | 5.6 | 44.5 | 5 | |
| Ceriops tagal | 10.8 | 9.9 | 20.7 | 11 | |||
| Lumnitzera littorea | 7.6 | 32.8 | 26.9 | 67.3 | 2 | ||
| Rhizophora apiculata | 26.0 | 21.2 | 34.7 | 12.1 | 42.3 | 136.2 | 1 |
| Rhizophora mucronata | 10.4 | 13.3 | 17.0 | 5.2 | 45.9 | 4 | |
| Rhizophora stylosa | 10.6 | 29.2 | 39.8 | 6 | |||
| Scyphiphora hydrophyllacea | 11.3 | 11.7 | 23.01 | 9 | |||
| Sonneratia alba | 21.9 | 6.3 | 28.2 | 8 | |||
| Sonneratia caseolaris | 64.1 | 64.1 | 3 | ||||
| Xylocarpus granatum | 14.6 | 14.6 | 13 | ||||
| Xylocarpus moluccensis | 13.6 | 21.2 | 4.1 | 38.9 | 7 | ||
| Total | 100 | 170 | 136 | 85 | 78 | 569 | |
| Rank | 3 | 1 | 2 | 5 | 4 | ||
Structural features e.g. the tree diameter at breast height (dbh), basal area, density and species richness differed among mangrove zones along the bay. Trees of larger dbh and higher species richness were found in zone 1 while those that comprised the highest basal area and density from zone 2 and zone 4, respectively (Table 4). Zone 1 is a fringing mangrove situated at Tiniguiban Cove while zone 2 is a riverine situated along the elevated landward portion of Sta Lucia and Iwahig River-estuaries. Zone 4 or the Irawan station includes an area planted with Rhizophora species thus tree density there was high, 8100 trees but of lower total basal area, only 106.22 m2/ha as opposed to at most 438.0 m2/ha from only 5800 tress at zone 2. Trees of intermediate dbh and basal were recorded from zone 3, which comprised the open coast fringing mangrove. Zone 5 is also situated in Tiniguiban Cove but unlike zone 1, it had a sparse mangrove distribution, only 800 trees/ha with comparable tree basal area due to the presence of a number of relatively huge Avicennia stands in this zone.
| Mangrove zone | No. of plots | Mean DBH (cm) | Basal Area (m−2 ha−1) | Density (tree ha−1) | Number of species |
|---|---|---|---|---|---|
| 1 | 11 | 10.2 (3.4)–104.5 (147.3) | 24.64–165.01 | 666–5200 | 4–15 |
| 2 | 13 | 5.9 (7.1)–28.1 (14.1) | 40.84–438.0 | 2166–5800 | 2–8 |
| 3 | 13 | 6.8 (2.8)–26.2 (31.9) | 36.71–176.89 | 3166–5450 | 6–10 |
| 4 | 4 | 9.6 (6.4) | 106.22 | 8100 | 8 |
| 5 | 4 | 90.8 (80.2) | 94.48 | 800 | 14 |
Human-resource interaction
In terms of anthropogenic disturbances on mangrove forest along Puerto Princesa Bay, zone 1 was found free from deforestation though TNG and SDW were more prone to garbage dumping due to their proximity to human settlement. Plastic bags, used fishing nets, and rubber strips were usually found entangled on prop roots and branches of mangroves in this zone. On the other hand, the Iwahig and Sta Lucia river-estuary portions were quite protected except for a few isolated areas where unregulated human access for occasional cutting and/or harvesting of Ceriops and Nypa was observed. The presence of Iwahig Community Tourism and Environment Association (ICTEA) and Iwahig Prison and Penal Farm somehow imposed protection measures on mangrove resource in the vicinity. In contrast though, the lower portion of the Iwahig riverbank was heavily populated. Presidential Proclamation No. 718 in 2004 allowed for segregation of parcel of land from Iwahig Prison and Penal Farm as civil reservation for resettlement and agricultural purposes, which later paved the way for establishment of “Bucana-Matahimik” resettlement project of the City of Puerto Princesa. Table 5 presents the count frequency of each human-related disturbance to mangrove forest as revealed by the key informants.
| Human-related disturbance | Frequency |
|---|---|
| Increased human population | 23 |
| Mangrove deforestation | 17 |
| Garbage dumping/improper waste disposal | 6 |
| Shellfish harvesting | 2 |
| Quarrying/fishpond construction | 1 |
| Soil erosion due to boat operation | 1 |
| Firefly watching/recreational activity | 1 |
Discussion
Species diversity
The mangrove forest of Puerto Princesa Bay is more diverse, with 28 mangrove species, compared to other surveyed mangrove sites in Palawan (PCSDS, 2006, 2004, 1999). The figure constitutes nearly 75% of the Philippine mangrove species. High diversity and cover of mangroves in the area would explain the occurrence of a wide array of mangrove-associated vertebrates (Dangan-Galon et al., unpublished) and invertebrates of mariculture importance (Dolorosa and Dangan-Galon, 2014).
True mangroves along the bay constitute 27 species following the classification of Tomlinson (1986). In a more recent classification made by Primavera et al. (2004), a slight discrepancy can be noticed. The authors considered Acanthus as true mangrove and Acrostichum as associate, which is opposite to that of Tomlinson (1986). Discrepancy on species categorization may depend greatly on the manner by which authors characterized the plant. Primavera and Subada used spatial distribution as basis to delineate Acrostichum as mangrove associate, occupying the landward portion of mangrove forest, which may be obvious in Panay Island, where the study was conducted. Tomlinson (1986) on the other hand categorized mangroves as major, minor element (regarded in this study as the true mangrove species), and associates on cosmopolitan basis. Accordingly, spatial distribution of Acrostichum is not limited to elevated sites but may be found interspaced with mangrove tress in disturbed areas, which is true to some surveyed sites along the Iwahig Riverbank.
An earlier report of 18 true mangrove species from the bay did not include Osbornia octodonta and Scyphiphora hydrophallacea as true mangroves rather mangrove associates (PCSDS, 2006) and therefore did not conform to the classification scheme of Tomlinson (1986). Regardless of such inconsistency, the present study remains to indicate the highest number of mangrove species recorded from the bay with five additional species, the A. marina, Avicennia officinales, Avicennia rumphiana, Aegiceras corniculatum, and Heritiera littoralis. These species were frequently found in San Pedro, Tiniguiban, and Iwahig River-estuary. These areas were not included in 2005 mangrove survey of PCSDS.
Vegetation types
Mangrove forest is structurally simple with only two vegetation types. A complex forest includes other vegetation e.g. the basin, over-wash, scrub, and hammock (Clough, 2013; Hogarth, 2007; Feller and Sitnik, 1996). The presence or absence of one vegetation type may be related not only to natural and anthropogenic disturbances but also to site’s geomorphology and hydrology (Urrego et al., 2014). As observed, tidal inundation, light intensity, and substrate composition have major influence on structural composition of mangroves along the bay although these variables were not measured empirically.
Human-resource interaction
Apparently, mangroves in some parts of the bay especially those in Iwahig and Sta. Lucia had remained intact over the years. The presence of fireflies, which serves as tourist attraction in Iwahig River-estuary, is an indicator of a pristine mangrove ecosystem. The observed disturbances may be recent and the adverse effects on species composition and structure are still insignificant. Forest conversion into fishpond, which is the major culprit to mangrove species loss or decline in the Philippines (Walters, 2004; Primavera and Esteban, 2008) is not rampant in the said stations. The presence of Iwahig Prison and Penal Farms in Iwahig-Sta Lucia probably imposed some sort of regulation on forest destruction in the vicinity.
At present though, sustainability of mangrove resource along the bay could not be ascertain. As mentioned, a portion of Iwahig riverbank had been converted into human resettlement. With increasing number of households residing in the bank, soil erosion, habitat destruction, and resource exploitation shall be inevitable in the near future.
Conclusion
Conservation effort, additional livelihood opportunities for the community, and information education campaign (IEC) are needed to protect the bay’s mangrove forest from expanding human population in the area. Protecting the mangrove forest and utilizing it sustainably could increase the coastal inhabitant’s resilience to foreseen impacts of climate change.
Acknowledgements
This research initiative is funded by the Commission on Higher Education through the R&D Program for Marine Biodiversity along Bohol and Sulu Seas and through the program leadership of Dr. Angel C. Alcala of the Silliman University-Angelo King Center for Research and Environmental Management (SUAKCREM). Recognition is also due to Palawan State University administration and to all research-affiliates and staff of Palawan State University-Marine Science Laboratory especially to Dr. Ramon C. Docto for sharing his data on community perception to physical changes in Iwahig mangrove ecosystem due to human interventions.
References
- Aliño et al., 2001 Aliño, P.M., Nañola, C., Roleda, M., Ticzon, V.S., 2001. Highlights of the assessment of the coastal habitats of Honda Bay, Palawan (2000–2001). Final Draft Report, Fisheries Resources Management Project. In: CD-ROM. Puerto Princesa City: Office of the City Planning and Development Coordinator.
- Becira, 2005 E.M. Becira State of mangroves in Tiniguiban Cove, Puerto Princesa Bay, Puerto Princesa City, Palawan. Sci. Diliman, 17 (2) (2005), pp. 46-51
- Clough, 2013 Clough, B., 2013. Continuing the Journey amongst Mangroves. ISME Mangrove Educational Book Series No. 1. International Society for Mangrove Ecosystems (ISME), Okinawa, Japan, and International Tropical Timber Organization (ITTO), Yokohama, Japan.
- Dolorosa and Dangan-Galon, 2014 R.G. Dolorosa, F.D. Dangan-Galon Species richness of bivalves and gastropods in Iwahig River-Estuary, Palawan, the Philippines. Int. J. Fish. Aquat. Stud., 2 (1) (2014), pp. 207-215
- Donato et al., 2011 Donato, D.C., Boone Kauffman, J., Murdiyarso, D., Kurnianto, S., Stidham, M., Kanninen, M., 2011. Mangroves among the most carbon-rich forests in the tropics. Letters published online/DOI: 10.1038/NGEO1123.
- English et al., 1997 S. English, C. Wilkinson, V. Bakers (Eds.), Survey Manual for Tropical Marine Resources, Australian Institute of Marine Science, Townville, Australia (1997)
- Feller and Sitnik, 1996 I.C. Feller, M. Sitnik Mangrove Ecology: A Manual for a Field Course. Smithsonian Institution, Washington, DC, USA (1996)
- Hogarth, 2007 P.J. Hogarth The Biology of Mangroves and Seagrasses. (second ed.), Oxford University Press, New York (2007)
- http//www.puertoprincesa.ph, 2012 http//www.puerto princesa city. gov.ph, 2012.
- Long and Giri, 2011 J.B. Long, C. Giri Mapping the Philippines’ mangrove forests using landsat imagery. Sensors, 11 (3) (2011), pp. 2972-2981
- McAleece et al., 1997 McAleece, N., Gage, J. D. G., Lambshead, P. J. D., Paterson, G. L. J., 1997. BioDiversity Professional statistics analysis software. Jointly developed by the Scottish Association for Marine Science and the Natural History Museum, London.
- PCSDS, 1999 PCSDS Coastal Resource Assessment: Roxas, Palawan. Palawan Council for Sustainable Development Staff, Puerto Princesa City, Palawan (1999)
- PCSDS, 2004 PCSDS Annual Accomplishment Report. Palawan Council for Sustainable Development Staff, Puerto Princesa City, Palawan (2004)
- PCSDS, 2006 PCSDS Baseline Report on Coastal Resources for Puerto Princesa City. Palawan Council for Sustainable Development, Puerto Princesa City, Palawan (2006), p. 130
- Primavera and Esteban, 2008 J.H. Primavera, J.M.A. Esteban A Review of Mangrove Rehabilitation in the Philippines: Successes, Failures and Future Prospects. Wetlands Ecol. Manage. (2008). DOI 10.1007
- Primavera and Sadaba, 2012 J.H. Primavera, R.B. Sadaba Beach Forest Species and Mangrove Associates in the Philippines. Southeast Asian Fisheries Development Center (SEAFDEC), Philippines (2012)
- Primavera et al., 2004 Primavera, J.H., Sadaba, R.B., Lebata, M.J.H., Altamirano, J.P., 2004. Handbook of Mangroves in the Philippines: Panay. Southeast Asian Fisheries Development Center (SEAFDEC), Philippines.
- Rönnbäck, 1999 P. Rönnbäck The ecological basis for economic value of seafood production supported by mangrove ecosystems. Ecol. Econ., 29 (1999), pp. 235-252
- Sheeran, 2006 K.A. Sheeran Forest conservation in the Philippines: a cost-effective approach to mitigating climate change?. Ecol. Econ., 58 (2006), pp. 338-349
- Sinfuego and Bout, 2014 K.S. Sinfuego, I.E. Bout Jr.Mangrove zonation and utilization by the local people in Ajuy and Pedada Bays, Panay Island, Philippines. J. Mar. Isl. Cult., 3 (2014), pp. 1-8
- Tomlinson, 1986 P.B. Tomlinson The Botany of Mangroves. Cambridge Tropical Biology Series, Cambridge, UK (1986)
- Urrego et al., 2014 L.E. Urrego, E.C. Molina, J.A. Suárez Environmental and anthropogenic influences on the distribution, structure, and floristic composition of mangrove forests of the Gulf of Urabá, Colombian Caribbean. Aquat. Bot., 14 (2014), pp. 42-49
- Walters, 2004 B.B. Walters Local management of mangrove forests in the Philippines: successful conservation or efficient resource exploitation?. Hum. Ecol., 32 (2) (2004), pp. 177-195
