Towards an ecosystem approach to small island fisheries: A preliminary study of a balanced fishery in Kotania Bay (Seram Island, Indonesia)
Fisheries and Marine Science Faculty, Pattimura University, Indonesia
Wageningen University and Research Centre, Netherlands
grace.hutubessy@yahoo.com
Fisheries and Marine Science Faculty, Pattimura University, Indonesia
Wageningen University and Research Centre, Netherlands
School of Arts and Social Sciences, Southern Cross University, Australia
Abstract
The Ecosystem Approach to Fisheries (EAF) is a holistic one as EAF considers all species as important elements within the eco-system. An EAF requires that community and ecosystem structure should be maintained by harvesting fish communities in proportion to their natural productivity, thereby sustaining the balance of species and sizes in a community. This article draws from research on the reef fish community and catch in Kotania Bay on Seram Island in Maluku, Indonesia, an area of approximately 6000 ha. Based on the trophic guild (ie the aggregation of species utilizing similar food resources) on the reef, the biomass of predator fish currently being captured now represents 40.4% of the total catch biomass. Members of the grouper family, the humphead wrasse (Cheilinus undulatus) and trevally (Caranx melampygus) in particular, have become targeted for sale in fish markets. If these predators are selectively targeted and exploited, the overall reef fishery and the human populations that depend on it may become imperilled, given these species’ significant roles in controlling those lower in the food chain. This study thereby emphasizes the need for balanced fisheries informed by the EAF model in small island fisheries management in order to sustain food security in such regions.
Keywords
Ecosystems, Balanced fisheries, Trophic guild, Small islands, Kotania Bay, Maluku
Introduction
Traditional fisheries management is largely based on single species stock assessment. Within this perspective the concept of ‘overfishing’, referring to loss of yield, has been recognized since the 1950s (Schaefer, 1954). It has, for instance, resulted in prescriptions against harvesting juveniles in order to allow fish to reproduce at least once before harvest (Sissenwine and Shepherd, 1987). Increasing concern about the small size of fish being captured in many fisheries has also led to improving selectivity in order to achieve a cleaner catch of “target species and size” with higher value (Broadhurst, 2008). While this single species assessment approach has had substantial impact on many fish stocks it has substantial shortcomings in that it focuses on isolated aspects (ie single species and size) rather than the function of size categories of species within a broader ecosystem. By contrast, the Ecosystem Approach to Fisheries (EAF) is a holistic management approach based on the entire ecosystem (including humans) (Pikitch et al., 2004; Bellido et al., 2011). Although he did not use the specific terminology, the need for an EAF was first broached in the 1930s and 1940s by Ricketts (1947, 1948) with regard to the fisheries of Monterey Bay in California and the viability of the area’s sardine fishery in particular. The goal of an EAF is to sustain marine ecosystems and the fisheries that occur within them in a productive, healthy and resilient condition sufficient to provide for human demand (www.fao.org/fi/glossary/default.asp). A key goal of the EAF that is particularly pertinent to our study is that of sustaining the structure of fish communities in marine eco-systems that are affected by fishing and by other land activities in coastal areas. Related to this, and with regard to areas where the eco-system has been substantially altered in recent times by (over-) fishing, Pitcher and Pauly (1998) also suggest the re-building of fish communities as one of the most important goals of fisheries management.
The goals of Indonesian fisheries management, as determined by the Indonesian Directorate General of Fisheries and the Ministry of Fisheries and Marine Affairs, revolve around the concept of Maximum Sustainable Yield (MSY). This approach was developed on an analysis of annual catch and effort data. Some arguments about the effectiveness of the MSY approach arose in Indonesia after indications of overexploitation by five Indonesian fisheries (Widodo, 2003) and the concept of MSY has proven to be ineffective in guiding fisheries management more generally (Mous et al., 2005). Scientific recommendations made by researchers from the Badan Riset Kelautan dan Perikanan (a marine research institution under the Ministry of Fisheries and Marine Affairs), including closing fishing grounds, limiting the issue of fishing licenses, creating minimum catch size rules and lowering fleet capacity, were offered to the government (Widodo, 2003). In response, in 2004, the Ministry of Fisheries and Marine Affairs issued a regulation (No. 45) regarding the minimum size of fish captured that was subsequently renewed in 2009. Once again, a single species based approach was applied to fisheries management without any means of monitoring and/or enforcing its provisions. As a result of ineffective policies and enforcement, Indonesian fisheries have faced certain depletion (Heazle and Butcher, 2007).
The existing crisis in Indonesian fisheries’ management, most manifest in western and central Indonesia, suggests that extending current approaches to the eastern part of the national archipelago is likely to replicate unsustainable fishing practices. In this regard, the national government’s designation of Maluku and North Maluku provinces as fish lumbung (a term that literally translates as ‘barns’ – implying an abundant resource) in 2010 and 2012 (respectively) is of particular concern. One aspect of the identification of regional lumbung is the establishment of marine protected areas (MPAs) within them in an attempt to compensate for increased fishing activity in the regions. While this represents a proactive approach to protecting fish stocks, previous experience with MPAs in other parts of Indonesia indicates that MPAs create conflict between communities, fishers, NGOs and industry groups (Morishita, 2008). Similarly, establishing lumbung areas without adequate management at the same time as increasing government subsidies to expand fishing fleets is highly problematic.
Fishing leads to a reduction in the abundance, biomass (Jennings et al., 1995) and mean size of species targeted by the fishery (Jennings et al., 1995). Increasing selective fishing pressure contributes to the truncation of age structure (Hsieh et al., 2006) and changes the composition of reef fish communities (Pinca, 2011). The impacts of fisheries can be detected in changing catch rates and catch composition (Welcomme, 1999). The management of a fishery requires a reliable prediction of the consequences of exploitation strategies (Sainsbury, 1982). A single-species fisheries management approach that avoids by-catch and other selectivity measures is an important tool to protect non-target and vulnerable species (Pikitch et al., 2004). However, improved selectivity may also lead to greater contrast in biomass among components of the trophic level. One common consequence of changes in trophic structures has been the depletion of apex predators (Polovina et al., 2009), intermediate consumers (Bundy et al., 2009) and predator fish (Hjermann et al., 2004). In this regard, management based on selective fishing tends to ignore the impact on the overall ecosystem (Garcia et al., 2012) and may not usefully support the end-goal of fishery management, which is to maintain the structure and function of the ecosystem (Zhou, 2008) as defined in the EAF (Garcia and Cochrane, 2005). A balanced fishery, which catches a selection of the natural population proportional to the productivity of its various size components (which is linked to trophic level) represents a model that may sustain fish production.
In this article, we aim to assess the condition of reef fisheries and of the reef fish community structure in a particular location based on our ongoing study of a balanced fishery in Kotania Bay. Kotania Bay hosts a type of traditional reef fishery common to Maluku and to Indonesia in general. Our study will address how traditional fishers utilize the fish resources in the bay, the effect of fisheries and how an ecosystem approach can be implemented to support regional food security in order to sustain the island community’s future.
Methods
Study sites
The ecosystems of Kotania Bay and the adjacent area of Pelita Jaya Bay are structured by mangroves, sea grass and corals (Fig. 1). Detailed studies undertaken in 1994 indicated that mangroves occupied 1250 ha, sea grass inhabited 115 ha and the coral that can be found along the bays covered 820 ha (Wouthuyzen & Sapulete, 1994) and there does not currently appear to be any significant variations. Fringing and atoll reefs are also scattered around the bay.
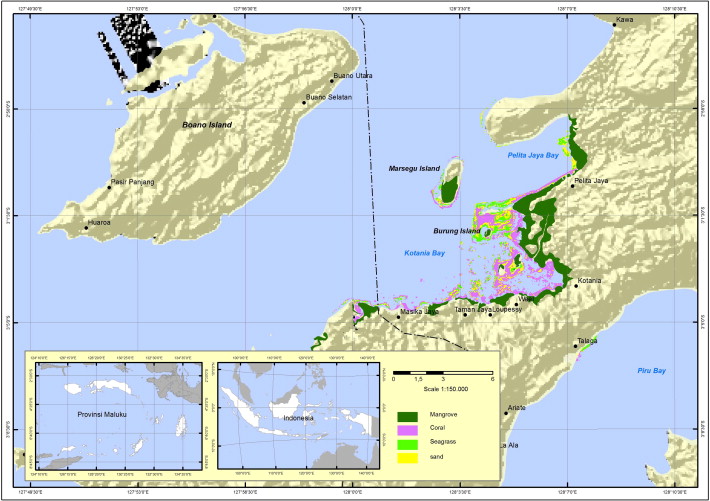
Fishing history
Due to a lack of detailed reef fisheries data from Kotania Bay, fisheries information was reconstructed from interviews with the fishers who live and catch reef fish around the bay (Neis et al., 1999). Fishers from six villages were interviewed to determine current and historic catch records (species and yields) and the types of fishing gear and methods that were used. Fishers were selected for interview in order to draw on a wide range of age and experience in the fishery. It was assumed that the 48 interviewed fishers were a representative sample of the 153 fishers who catch (mainly reef) fish in Kotania Bay (Anon., 2012). Data was tabulated per decade to identify trends of the catch rates. While fisheries statistics for West Seram Bay was available from 1980 to 2012, similar data on the specific Kotania Bay area was only available from 2012 onward. We estimated relevant fisheries data for Kotania Bay in previous decades by extrapolating from the 2012 data.
Surveying fish communities
Two divers and one boatman were employed to conduct this study. At each contiguous reef site, fish species biomass was recorded on a 20 m × 4 m transect. On shallow and dense coral reef patches, a stationary point count was conducted around a 5 m radius. This was a shortening of the 10 m radius point count advocated by Labrosse et al. (2002) in order to analyze crowded reef fish areas (including small species) in the range of visibility. The survey was conducted at depths of 0.5–20 m covering the whole area of the bay. All fish that were present during those periods were recorded, with the exception of small cryptic species (e.g., blennies and gobies) that are difficult to record. During the survey of fish communities the observers remained stationary or moved slowly after their 5 m dives, thereby reducing the likelihood of frightening fish into or out of the transect. Individual fish that moved along transects during surveying were recorded once only. The observer recorded the scientific names of fish, number of fish per species and estimated their size. Every species recorded was grouped according to their trophic level (Froese and Pauly, 2004).
Catch
Catch data was gathered from creel surveys and logbooks completed by fishers between June and December 2012. Creel surveys (Lockwood, 2000) involve estimations of fishers’ catches (derived from interviews with fishers) along a transect and were conducted to determine how many fish are being caught and kept by reef fishermen during fishing hours (night and day) in Kotania Bay. The gear used to harvest reef fish comprises gillnets and traps Species type, length and weight, number of individuals and trophic level were used to estimate reef fish biomass.
Balanced fishery
The link between exploitation rate and natural productivity was analyzed from the biomass-size spectrum in the community of reef fish and their size distribution of catch. The biomass of fishes (g 100 m−2), aggregated regards of trophic guild, were normalized by regressing the log10 of the biomass of 1 cm interval class sizes against log10 of the size classes. By distributing the biomass density size independently to the size interval, comparing results from different communities, whatever the size of the organisms, is possible (Blumenshine et al., 2000). The regressions were performed separately for fish biomass and the catch. Two slopes of logarithms of catch (kg) and observed biomass over log-length were compared using a simple Student’s t-test method for testing hypotheses about the equality of (Zar, 2002). The percentage of different trophic guilds contributing to the total biomass for the fish community and catch was calculated. The five trophic levels that were used included omnivores, zooplanktivores, zoobenthivores, herbivores and piscivores – following Froese and Pauly, 2004.
Results
Fishing pressure
Kotania Bay appears to have been exploited since the 1950s. Small gillnets were initially used as the easiest means of catching pelagic fish in small numbers. This type of fishing continued until the 1980s when this area became regarded as a reef fishing area. Some old fishers also used bamboo fish traps and bamboo barrier nets to catch fish for personal subsistence purposes. Since the early 2000s, improved road networks in West Seram have resulted in local marine resources becoming valuable and economically important. Around this time some professional fishers started using a variety of gear, such as handlines, longlines and spear guns in the area, working from wooden or fiberglass boats with small engines (around 5.5 HP). The latest fishing gear used to catch coral trout is the trolling line, which consists of wire and a lure. Destructive illegal fishing methods such as bombs and poison have been used for many years but very few of the individuals using them have been apprehended. Most fishers acknowledge the negative impact of the latter practices on fish resources but there is no sign of the practice stopping.
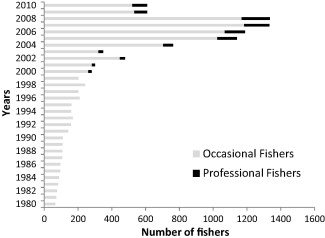
The number of individuals involved in catching reef fish in Kotania Bay has varied considerably over the past three decades. In between 1980 and 1999 the number gradually increased from 65 to 242 (Fig. 2). Decreases in 2000 and 2003 might be related to population movements related to the social conflict that occurred in Maluku in 1999 (which continued until 2002) and its aftermath (see Duncan, 2013). The number of fishers increased to 478 by 2004 and doubled again by 2008. The marked reduction in number of fishers after 2009 indicates the depletion of marine resources such as sea cucumber (Holothurian) and sharks.
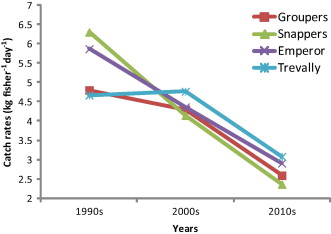
Catch rates for reef fish in Kotania Bay show a steady decline over the period between the 1990s and 2010s. In total, catch rates for targeted species such as grouper, snapper, trevally and emperor, were 5.4 kg fisher−1 day−1 in 1990s. Since this peak, the volume has decreased steadily, reaching 2.7 kg fisher−1 day−1 in 2010s. Interestingly, this pattern of decline differs slightly with regard to species composition, with different rates of decline demonstrating a lack of synchrony with each other (Fig. 3).
The reef fish community
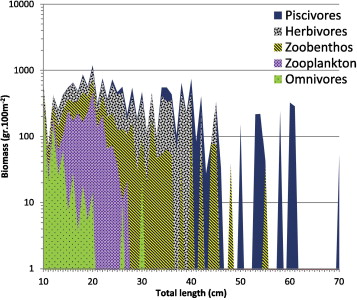
The total number of fish observed during the diving surveys was 40,111 along 45 transects, consisting of 20 families and 312 species with total estimated biomass of 659.5 kg. Mean density and biomass of reef fish per transect was 22.3 fish m−2 and 183.2 g m−2, respectively. Five families were ubiquitous throughout the study sites: Pomacentridae, Caesionidae, Labridae, Scaridae, and Acanthurdae. The most abundant species were damsel fish (Chromis viridis, Pomacentrus amboinensis, Amblyglyphidodon curacao), fusilier (Caesio cunning) and cardinal fish (Apogon neotes). Throughout the coral habitat in the bay, density and biomass were dominated by piscivorous species, which accounted for 30.3% of the total fish counted, followed by herbivores (29.7%) and zoobenthivores (28%). Only 9% of the fish counted were omnivores and 14% of zooplanktivores (Fig. 4). The calculation of slope of size spectrum in the community was −0.88, indicating decreased biomass with size. The large size range of herbivores was dominated by parrotfish (Scarus oviceps and Scarus ghobban); and Napoleon fish (Cheilinus undulatus) while coral trout (Plectropomus oligocanthus) dominated the large carnivore and predator groups, respectively.
Catch composition
One hundred and fifty-one species were caught during this study with the composition being 43% piscivore, 26.4% zooplanktivore and zoobenthivore, 9.1% omnivore and 21.5% herbivore. Piscivorous fish caught comprised 34 species, with long-finned rockcod (Epinephelus quoyanus) being the most frequently caught. Monocle breams (Pentapodus trivittatus and Scolopsis bilineatus) were the most abundant among the 34 zoobenthivores species. Herbivorous fish consisted of 40 species, which were dominated by rabbitfish (Siganidae) and parrotfish (Scaridae). Fish size distribution of the multi-species, multi-gear catch in Kotania showed a similar pattern among the trophic group. The negative distribution tended to be skewed, reaching a peak of about 30 cm (Fig. 5). The maximum size of herbivores was about 40 cm, zoobenthivores about 54 cm and piscivores were more than 100 cm. The largest species was barracuda (Sphyraena jello (109 cm SL). The slope of catch was −1.125 while the slope of the observed biomass spectrum in dive surveys was −0.878. One-tailed testing on the slope of biomass-size spectrum and slope of catch indicates minimal difference (P = 0.58), suggesting that the two slopes are parallel. Parallelism between natural biomass and catch biomass could be considered as a balanced harvesting if we assume that the size structure of the fished community is the same as the observed community through the dive surveys (Law et al., 2012).
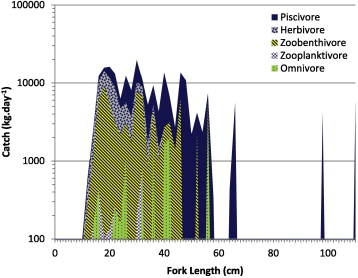
The composition of herbivorous fishes in the reef fish community was 41.9%, which only represented 17% of the total biomass caught. In the natural fish population small size species dominated the zoobenthivores biomass while the bigger size species dominated the catch.
Discussion
As Zeller et al. (2006) identified (with regard to another regional fishery, American Samoa 1950–2002), a variety of factors can influence local socio-economic systems and related behavior and thereby influence fisheries. The interaction of these factors is often complex. The social tensions and inter-communal violence that occurred in some urban areas of Maluku in 1999–2001 prompted a substantial exodus from the region. In the case of Kotania Bay, many recently arrived individuals and/or families returned to their original villages in South and Southeast Sulawesi. This resulted in an immediate decline in the number of fishers working in the bay. When the conflict ended in 2001 previous levels of fishing did not resume in the same manner but rather responded to new factors. The immediate post-conflict period saw a diversification of employment opportunities that arose from increased demand for spices and natural medicines, creating jobs in harvesting crops such as eucalyptus, cacao, and seaweed. Further diversification of employment options also began after the establishment of West Seram Region in 2004. While these options gave the local population opportunities for economic advancement outside of fishing, a counter-tendency was also present in that increases in demand for particular reef fish in urban centers created an increased income for those people still employed in fishing activities in Kotania Bay. Substantial income was generated by fishing, processing and marketing and reef fish culture in floating cages also increased to provide live fish for the international market (LIPI Report, 2008). The establishment of set prices per kilo for reef fish by the national government in 2007 prompted a number of young adults to become occasional fishers harvesting groupers (Serranids). As a result, fishing pressure in the bay (including the harvesting of sea cucumbers) increased until 2008, resulting in a significant depletion of marine resources. The reduction in fishers in 2009 resulted from both this depletion and from employment opportunities arising from major infrastructural projects such as roads, bridges and ports in the area.
Harmful unselective fishing practices in Kotania can be perceived from the diversity of catch in terms of species and size. With regard to the 151 species fished, the catch composition was dominated by piscivorous fish, followed by herbivorous fish. Piscivorous and zoobenthivores fish are mostly caught by line fishing, which represents the predominant method used in the bay (using different hook sizes). Herbivorous fish were caught by traps and gillnets. Traps are recognized as less selective gear (Hawkins et al., 2007), capturing a wide range of sizes and species (line fishing and gillnets catch a specific range of sizes depending on the mesh size and size of hook used). Given this, we may then assume that there will be a phase shift from piscivorous and herbivorous to another trophic guild in the community. However, these two trophic levels were dominant in the community structure in the bay. Jennings and Polunin (1998) suggested that harvesting a range of species from various trophic groups might produce high yield without initiating ecosystem shift. Although the multiple forms of gear used in the bay have resulted in unselective fishing (catching a wide range of sizes and species and with a limited amount of unwanted by-catch being discarded) this appears to have had little impact on the community structure (van Zwieten, 2003, 2010). However the impact of bombing of reefs, a practice still present in Kotania Bay, has not been addressed yet. By contrast, selective fishing of herbivorous fishes, parrotfish and surgeonfish in the Great Barrier Reef created a shift from coral to macroalgae dominance and an invertebrate feeder shift to eating macroalgae (Bellwood et al., 2006). Reducing the population of specific target species will affect species interactions in the ecosystem (Bundy et al., 2005), where predation by non-target species from higher tropic level increased and competition for carrying capacity at the same tropic level decreased (Zhou, 2008).
Drawing on the above, can we evaluate whether reef fisheries in Kotania Bay are more or less balanced in terms of fish exploitation? Rochet et al. (2011) suggested measuring the effects of a size-selectivity curve (which depends on size selection and the community size structure) on species-size diversity. Our result showed the wide range of fish size (shown in Fig. 5) represented multi-species selectivity from the multiple gears used. The high biomass of large fish caught might indicate of large size targeting in the fishery which also shown by the slope of catch size-spectrum. A negative skew of biomass distribution, dominated by fish sizes of less than 30 cm might indicate a high level of productivity of these classes. In comparison to the size spectrum of the fish community in Kotania Bay, the volume of fish harvested in the bay is consistent with the available productivity but is less proportional because large fish (±100 cm) were absent during the census, which nevertheless does not mean that no large fish are available in the community. Limited depth range during underwater visual censuses constrains the observation of large fish. It is premature to answer the above question with the assessment that fishing activities in Kotania Bay suggest a balanced fishery. Given that each species and size is vulnerable to a particular type of fishing gear, an increase in intensity of fishing using a particular gear may impact the ecosystem. The study found that the proportion of fish caught is significantly different for herbivorous and zoobenthivores fishes. Use of other types of gear might allow balanced fisheries to be performed in the bay. With regard to the size-spectrum slope in fisheries management, Garcia et al. (2012) suggested that steepening or flattening of the slope might be an indicator of fishing impact on the reef fish community (also see Graham et al., 2005).
Fishing disturbance results in a steeper slope of biomass size spectrum in selective targeting of larger individuals in the community (Hall et al., 2006). In the context of Kotania Bay, we only provided a community size structure and trophic level in one space and time (and no other scenarios are available for comparison). The result of a steeper slope (b = −0.88) compared to the catch may indicate that the biomass of large body size has been affected. Species that have a large body size, as a general rule, tend to achieve maturity later, have lower rates of rapid potential population increase and experience larger population declines in response to fishing (Jennings et al., 1999). There is also a substantial concern that taking mainly the large and mature fish will affect egg quality and larval survival (Hsieh et al., 2006), may generate an evolutionary effect on body growth in the long term and might also influence the system stability and decrease its size diversity (Rochet et al., 2011). If these factors have occurred in Kotania Bay, where selective fishing targeted on large fishes has been high, the future population will be composed of smaller sized species of fish, a pattern experienced in other fishing areas (Pet-Soede et al., 2001; Muljadi and Hehuat, 2012). Due to the scarcity of fishing activities recorded in Kotania Bay, there are concerns over the judgment of selectivity fishing practices. Further studies regarding spatial and temporal size spectrums in the community of reef fish in the bay resulting from the impact of fisheries are essential.
The results of our study lead us to advocate concepts of EAF and balanced harvesting for small island fisheries and a series of approaches to securing this. The first priority is to establish both open and restricted fishing areas. While Kotania Bay does not have a designated no-fishing zone under an EAF system, part of the bay is effectively a no-fishing area reserved for the pearl culture industry. An (unpublished) census we undertook on reef fish in the pearl culture area showed that larger fish were abundant. The relative densities, composition and movements of reef fish in and across the boundary are unknown due to no clear boundaries being set up, however, we hypothesize that a ‘spillover’ of fish may be occurring from the cultured area (McClanahan and Mangi, 2000) to the benefit of the bay fishery in general. Further, a detailed study is needed to examine this hypothesis in order to support the Maluku government’s implementation of the National Fish Barn program, involving the establishment of restricted fishing areas.
Although we acknowledge the limitations of our research project, results and analysis, our study supports the balance fishery perspective identified by Garcia et al. (2012), in that harvesting fish proportionally with natural levels of productivity creates less disturbance of community structure than other approaches. In order to develop EAF we need to expand our knowledge of the selective impacts of fishing gears and exploitation strategies on reef fish at the community scale.
Acknowledgement
Thanks to Paul Butcher for his feedback on an earlier draft of this article.
References
- Anon., 2012 Anon.Daftar nama penduduk Kabupaten Seram Bagian Barat yang memiliki Kartu Tanda Penduduk dengan status nelayan. Dinas Kependudukan dan Catatan Sipil Kabupaten SBB, Maluku (2012)
- Bellido et al., 2011 J.M. Bellido, M.B. Santos, M. Grazia Pennino, X. Valeiras, G.J. Pierce Fishery discards and bycatch: solutions for an ecosystem approach to fisheries management?. Hydrobiologia, 670 (2011), pp. 317-333
- Bellwood et al., 2006 D.R. Bellwood, T.P. Hughes, A.S. Hoey Sleeping functional group drives coral-reef recovery. Curr. Biol., 16 (2006), pp. 2434-2439
- Blumenshine et al., 2000 S.C. Blumenshine, D.M. Lodge, J.R. Hodgson Gradient of fish predation alter body size distribution of lake benthos. Ecology, 81 (2000), pp. 374-386
- Broadhurst, 2008 M.K. Broadhurst Working laterally towards perfect selectivity in fishing gears. Am. Fish. Soc. Symp., 49 (2008), pp. 1303-1309
- Bundy et al., 2005 A. Bundy, P. Fanning, K.C.T. Zwanenburg Balancing exploitation and conservation of the eastern Scotian Shelf ecosystem: application of 4D ecosystem exploitation index. ICES J. Mar. Sci., 62 (3) (2005), pp. 503-510, 10.1016/j.icejms.2004.12.008
- Bundy et al., 2009 A. Bundy, J.J. Heymans, L. Morisette, C. Savenkoff Seals, cod and forage fish: a comparative exploration of variations in the theme of stock collapse and ecosystem change in four Northwest Atlantic ecosystems. Prog. Oceanogr., 81 (2009), pp. 188-206
- Duncan, 2013 C. Duncan Violence and Vengeance: Religious Conflict and Its Aftermath in Eastern Indonesia. Cornell University Press, New York (2013)
- Froese and Pauly, 2004 Froese R., Pauly, D. (Eds.), 2004. FishBase. Catalogue of Life Fish. <www.fishbase.com>.
- Garcia and Cochrane, 2005 S.M. Garcia, K.I. Cochrane Ecosystem approach to fisheries: a review of implementation guidelines. ICES J. Mar. Sci., 62 (2005), pp. 311-318
- Garcia et al., 2012 S.M. Garcia, J. Kolding, J. Rice, M.-J. Rochet, S. Zhou, T. Arimoto, J.E. Beyer, L. Borges, A. Bundy, D. Dunn, E.A. Fulton, M. Hall, M. Heino, R. Law, M. Makino, A.D. Rijnsdorp, F. Simard, A.D.M. Smith Reconsidering the consequences of selective fisheries. Science, 355 (2012), pp. 1045-1047
- Graham et al., 2005 N.A.J. Graham, N.K. Dulvy, S. Jennings, N.V.C. Polunin Size-spectra as indicators of the effects of fishing on reef fish assemblages. Coral Reef, 24 (2005), pp. 118-124
- Hall et al., 2006 S.J. Hall, J.S. Collie, E.D. Duplisea, S. Jennings, M. Bravington, J. Link A length-based multispecies model for evaluating community responses to fishing. Can. J. Fish. Aquatic Sci., 63 (2006), pp. 1344-1359
- Hawkins et al., 2007 J.P. Hawkins, M. Callum, C.M. Roberts, F.R. Gell, C. Dyth Effects of trap fishing on reef fish communities. Aquat. Conserv. Mar. Freshwater Ecosyst., 17 (2007), pp. 111-132
- Heazle and Butcher, 2007 M. Heazle, J.G. Butcher Fisheries depletion and the state in Indonesia: towards a regional regulatory regime. Mar. Policy, 31 (2007), pp. 276-286
- Hjermann et al., 2004 D.Q. Hjermann, G. Ottersen, N.C. Stenseth Competition among fishermen and fish causes the collapse of Barents sea capelin. Proc. Natl. Acad. Sci. U.S.A., 101 (2) (2004), pp. 11679-11684
- Hsieh et al., 2006 C.H. Hsieh, C.S. Reiss, J.R. Hunter, J.R. Beddington, R.M. May, G. Sugihara Fishing elevates variability in the abundance of exploited species. Nature, 443 (2006), pp. 859-862
- Jennings et al., 1995 S. Jennings, E.M. Grandcourt, N.V.C. Polunin The effect of fishing on the diversity, biomass and trophic structure of Sheychelles’reef fish communities. Coral Reef, 14 (1995), pp. 225-235
- Jennings et al., 1999 S. Jennings, S.P.R. Greenstreet, J.D. Reynolds Structural changes in an exploited fish community: a consequence of differential fishing effects on species with contrasting life histories. J. Anim. Ecol., 68 (1999), pp. 617-627
- Labrosse et al., 2002 P. Labrosse, M. Kulbicki, J. Ferraris Under Water Visual Fish Census Survey. Proper Use and Implementation. Secretariat of Pacific Community, New Caledonia (2002)
- Law et al., 2012 R. Law, M.J. Plank, J. Kolding On balance exploitation of marine ecosystem: result from dynamic size spectra. ICES J. Mar. Sci. (2012), 10.1093/icesjms/fss031
- LIPI Report, 2008 LIPI Report, 2008. Pemberdayaan dan peningkatan pendapatan nelayan melalui usaha perikanan terpadu di teluk Kotania. UPT Balai Konservasi Biota Laut Ambon, Pusat Penelitian Oseanografi, LIPI. 41 hal.
- Lockwood, 2000 S.J. Lockwood The effect of fishing on marine ecosystem and communities. Aquat. Conserv. Mar. Freshwater Ecosyst., 10 (3) (2000), pp. 226-227
- McClanahan and Mangi, 2000 T.R. McClanahan, S. Mangi Spillover of exploitable fishes from a marine park and its effect on the adjacent fishery. Ecol. Appl., 10 (2000), pp. 1792-1805
- Morishita, 2008 J. Morishita What is the ecosystem approach for fisheries management?. Mar. Policy, 32 (2008), pp. 19-26
- Mous et al., 2005 P.J. Mous, J.S. Pet, Z. Arifin, R. Djohani, M.V. Erdmann, A. Halim, M. Knight, L. Pet-Soede, G. Wiadnya Policy needs to improve marine capture fisheries management and to define a role for marine protected areas in Indonesia. Fish. Manage. Ecol., 12 (2005), pp. 259-268
- Muljadi and Hehuat, 2012 Muljadi, A.H., Hehuat, Y., 2012. Status populasi ikan komersial di Kepulauan Banda. In Kajian Cepat Kelautan Kepulauan Banda, Maluku Tengah. Coral Triangle Centre, Laporan 2012. 163pp.
- Neis et al., 1999 B. Neis, D.C. Schneider, L. Felt, R.L. Haedrich, J. Fischer, J.A. Hutchings Fisheries assessment: what can be learned from interviewing resource users?. Can. J. Fish. Aquatic Sci., 56 (1999), pp. 1949-1963
- Pet-Soede et al., 2001 C. Pet-Soede, W.L.T. van Denses, J.S. Pet, M.A.M. Machiels Impact of Indonesian coral reef fisheries on fish community structure and the resultant catch composition. Fish. Res., 51 (2001), pp. 35-51
- Pikitch et al., 2004 E.K. Pikitch, C. Santora, A. Babcock, A. Bakun, R. Bonfil, D.O. Conover, P. Dayton, P. Doukakis, D. Fluharty, B. Heneman, E.D. Houde, J. Link, P.A. Livingston, M. Mangel, M.K. McAllister, J. Pope, K.J. Sainsbury Ecosystem-based fisheries management. Science, 305 (2004), pp. 346-347
- Pinca, 2011 S. Pinca, M. Kronen, F. Magron, B. McArdle, L. Vigliola, M. Kulbicki, S. Andréfouët Relative importance of habitat and fishing in influencing reef fish communities across seventeen Pacific Island Countries and Territories. Fish Fish. (4) (2011), pp. 361-379
- Pitcher and Pauly, 1998 Pitcher, T.J., Pauly, D., 1998. Rebuilding ecosystem, not sustainability, as the proper goal of fisheries management. In: Pitcher, T., Pauly, D., Hart, P. (Eds.), Reinventing Fisheries Management, pp. 311–325 (Chapter 24). Chapman & Hall Fish and Fisheries Series. 435pp.
- Polovina et al., 2009 J.J. Polovina, M. Abecassis, E.A. Howell, P. Woodworth Increases in the relative abundance of midtrophic level fishes concurrent with declines in apex predators in the subtropical North Pacific, 1996–2006. Fish. Bull., 107 (4) (2009), pp. 523-531
- Ricketts, 1947 Ricketts, R., 1947. ‘Science studies the Sardine’, Monterey Peninsula Herald (Sardine Edition) n12 March 7th: 1, 3 (archived online at: http://www.datadeluge.com/2009/06/science-studies-sardine-mysterious_27.html).
- Ricketts, 1948 Ricketts, R., 1948. ‘Scientist report on sardine supply’, Monterey Peninsula Herald (Sardine Edition) n13 April 3rd: 1, 3.
- Rochet et al., 2011 M. Rochet, J.S. Collie, S. Jennings, S.J. Hall Does selective fishing conserve community biodiversity? Prediction from a length-based multispecies model. Can. J. Fish. Aquatic Sci., 68 (2011), pp. 469-486
- Sainsbury, 1982 Sainsbury, K.J., 1982. The ecological basis of tropical fisheries management. In: Pauly, D., Murphy, G.I. (Eds.), Theory and Management Tropical Fisheries. In: Conference Proceedings, ICLARM, Manila, Philippines, vol. 9, pp. 167–188.
- Schaefer, 1954 M.B. Schaefer Some aspects of the dynamics of populations important to the management of the commercial marine fisheries. Bull. Inter-Am. Trop. Tuna Comm., 1 (1954), pp. 27-56
- Sissenwine and Shepherd, 1987 M.P. Sissenwine, J.G. Shepherd. Can. J. Fish. Aquatic Sci., 44 (1987), p. 913
- Welcomme, 1999 R.L. Welcomme A review of a model for qualitative evaluation of exploitation level in multi-species fisheries. Fish Manage. Ecol., 6 (1999), pp. 1-19
- Widodo, 2003 J. Widodo Pengkajian stok sumberdaya ikan laut Indonesia tahun 2002 [Review of Indonesia’s marine fishery 2002]. J. Widodo, N.N. Wiadnyana, D. Nugroho (Eds.), Prosiding forum pengkajian stok ikan laut di perairan Indonesia, Jakarta, 23–24 Juli 2003, PUSRIPT-BRKP, Ministry of Marine Affairs and Fisheries, Jakarta (2003), pp. 1-12
- Wouthuyzen & Sapulete, 1994 Wouthuyzen & Sapulete Keadaan wilayah pesisir di Teluk Kotania, pada waktu lalu dan sekarang: Suatu tinjauan. Perairan Maluku dan sekitarnya, 7 (1994), pp. 1-18
- Zar, 2002 J.H. Zar Biostatistical Analysis. (3th ed.), Prentice-Hall International Editor, Inc., Englewood, New Jersey (2002)
- Zeller et al., 2006 D. Zeller, S. Booth, P. Craig, D. Pauly Reconstruction of coral reef fisheries catches in American Samoa, 1950–2002. Coral Reefs, 25 (2006), pp. 144-152
- Zhou, 2008 S. Zhou Fishery by-catch and discards: a positive perspective from ecosystem-based fishery management. Fish Fish., 9 (2008), pp. 308-315
- van Zwieten, 2003 van Zwieten, P.A.M., Goudswaard, P.C., Kapasa, C.K., 2003. Mweru-Luapula is an open exit fishery where a highly dynamic population of fishers makes use of a resilient resource base. In: E. Jul-Larsen, J. Kolding, R. Overa, J. Raakjaer Nielsen, P.A.M. van Zwieten (Eds.), Management, Co-management or No-management? Major Dilemmas in Southern African freshwater fisheries, FAO Fisheries Technical Paper 426/2. FAO, Rome.
- van Zwieten, 2010 van Zwieten, P.A.M., Bene, C., Kolding, J., Brummet, R. (Eds.), 2010. ‘Review of tropical reservoir and their fisheries in developing countries: the cases of the Lake Nasser, Lake Volta and Indo-Gangetic basin reservoir’, with the contribution of E.K. Abban, H. Adam, K. Agboga, H.R. Dankwa, O. Habib, P. Katiha, I. Omar, M. Sherata, H.A.R. Soliman, K.K. Vass, M. Zaki. FAO Fisheries Technical Paper. Rome.
