Composition and diversity of Mangrove species in Camotes Island, Cebu, Philippines
Abstract
Mangrove forests are one of the world’s most threatened tropical ecosystems. The study aimed of assessing the composition and diversity of Mangrove species in Camotes Island, Philippines, as basis for its conservation and protection. A Belt in line transect method was used, with a minimum length of 1000m and maximum of 2000m along landward, middle, and seaward at an interval of 100m. Quadrat of 10 x 10m were established within the transect line at an interval of 250m. All the plant species within the quadrats were identified and measured.
A total of 42 mangrove species were recorded in the island, representing 31 true mangrove species, and 11 mangrove associates. Two species were identified as Endangered Pemphis acidula J.R.Forst. & G.Forst and Camptostemon philippinense (S.Vidal) Becc, with a relative value of high species diversity (H’ = 3.0107). Camotes Island were dominated by the species of Sonneratia alba Sm, Avicennia marina (Forssk.) Vierh Avicennia rumphiana Hallier f, Rhizophora apiculata Blume, and Rhizophora stylosa Griff. Jaccard’s and Sorensen dissimilarity matrix prove that the mangrove species of Camotes Island were forming three cluster.
Conservation effort and Information Education Campaign were needed by the Local Government Units, to protect the high mangrove species diversity of Camotes Island.
Keywords
Composition, diversity, Pilar, Poro, San Francisco, Tudela
Introduction
Mangrove forests are one of the world’s most threatened tropical ecosystems, and in fact 11 true mangrove species are already in the IUCN Red List (Polidoro et al., 2010). As reiterated by Giri et al. (2010), Mangrove forest throughout the world has declined from 18.8 million hectares in 1980 to 15.2 million hectares in 2005. Based on the record of the Food and Agricultural Organization of the United Nations (FAO-UN, 2015) Asia has the most extensive mangrove forest cover, but with the most serious deforestation rates. In Thailand, mangrove forest is already fragmented due to the combined effect of natural disasters and anthropogenic disturbances (Doydee and Buot, 2010).
In the Philippines the rate of losing mangroves is up to 50% (Maritime Review, 2017). As a result, more number of mangrove forest are on the brink of complete collapse (Gevaña, Pulhin, & Tapia, 2019). Based on the record of IUCN, mangrove species in the Philippines are in danger of extinction due to coastal development, climate change, logging, industry, settlements, and agriculture (Maritime Review, 2017). In Cebu Island, anthropogenic activities were also the culprit of mangrove destruction. Coastal inhabitant uses mangrove tree species for crafting poles, fencing, forage, and house construction, as well as for fuel wood (Lillo and Buot, 2016). Mangrove forest also serve as potential sources of livelihood that can provide incentives to coastal communities (Camacho et al., 2011).
The Camotes Island Mangrove Swamp Forest Reserve is declared as protected area under Proclamation No. 2152, s. 1981. The declaration as protected area cover the coastlines of Camotes Island, which serve as a vital defense against the impacts of climate change. Mangroves are important and valuable, especially on their buffering capacity against storm surge, tsunami, and flooding (Macintosh, 2010; Garcia, Malabrigo & Gevaña, 2014; Gevaña, Camacho, & Pulhin, 2018). Majority of the inhabitants of Camotes Island are residing within 60 km from the coastal zones, making them exposed to natural disasters (PSA, 2017).
Camacho et al. (2011) emphasizing also that mangrove forest plays an important role in Carbon sequestration. Based on the study of Aksornkoae & Kato (2011), a 40-year-old mangrove plantation sequesters 370.7 tons of Carbon per hectare, then a 15-year-old plantation sequesters 208.5 tons of Carbon per hectare. While a 20-year-old plantation sequesters 149.5 tons of Carbon per hectare, and a natural stand sequesters 145.6 tons of Carbon per hectare, lower as compared to established plantation. In addition, mangrove plantation establishment was also driven by the increasing demand for fuelwood, poles, charcoal and woodchips, as well as on their ecological ecosystem functions (Aksornkoae & Kato, 2011).
In the Philippines the target area for reforestation totals to 1.5 million hectares including mangroves to be planted by 1.5 billion trees of combined forest species and fruit trees, with a total budget of 5 billion pesos for a period of six years (DENR-NGP Website). However, despite this reforestation and afforestation effort of the government, still forest ecosystems experience a poor ratio between forest loss to forest gain (3.6:1 from 2000–2010 period) (Hansen et al., 2013). Planting efforts implemented were unsuccessful due to the lack of science-based approach guidelines (López-Portillo et al., 2017). In this situation, there is an urgent need for enhancing our silvicultural knowledge about mangroves, as the key to increase the effectiveness and efficiency of rehabilitation (Thompson, Clubbe, Primavera, Curnick, and Koldewey, 2014).
Philippines serve as habitat to 39 true mangrove species (Sinfuego and Bout, 2014). In Palawan Island almost all Mangrove ecosystems in each Municipalities were documented. Palawan Island has 23 true mangrove species, with species richness ranges from 8 to 17 per Municipality (Dangan-Galon et al., 2016). In Cebu Island information on mangrove species in terms of its composition and diversity were still in adequate. The latest available reference was in Argao, Cebu with 22 species of true mangrove (Lillo and Buot, 2016), and nothing in Camotes Island in any scientific journals.
Knowing the exact composition of mangroves species in Camotes Island was an important prerequisite in understanding its ecology and ecosystem functioning. The information of the mangrove species serve as an important input to the conservation of all marine biodiversity in the island (Lillo and Buot, 2016). Sustainability of natural resources was always intimately linked to ecology. The study aimed of assessing the composition and diversity of Mangrove species in Camotes Island, Philippines, as basis for its conservation and protection.
Methodology
Study area
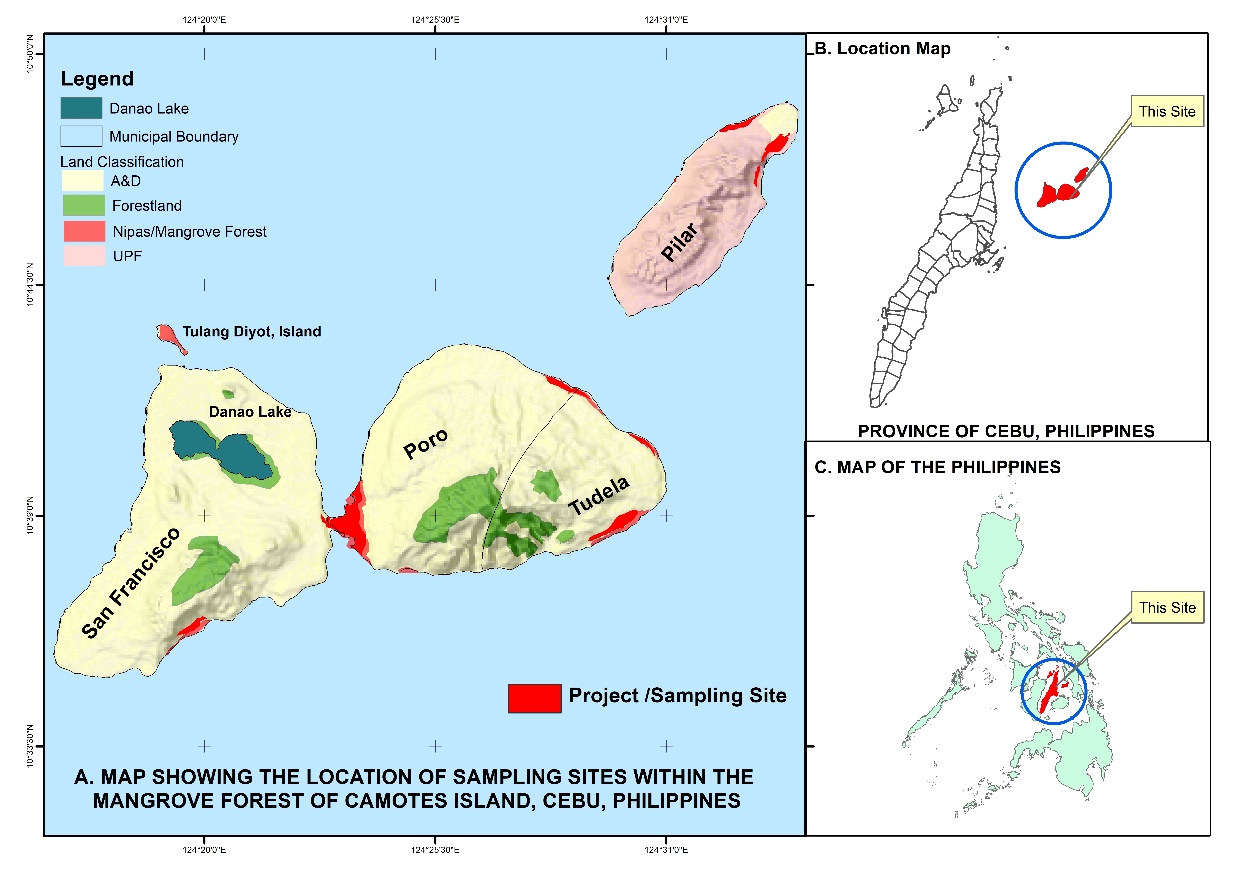
Camotes Island is found off the north eastern coast of the Island of Cebu, and lies at a coordinates of 10˚37′ to 10º43′10″N, and 124º24′40″ to 124º29′4″E (Figure 1). Camotes Island comprises three major Islands namely; Pacijan, Poro and Ponson. Pacijan Island is entirely within the political jurisdictions of the Municipality of San Francisco (Figure 1). While the whole Island of Ponson belong to the Municipality of Pilar. Then the Municipalities of Poro and Tudela are located on Poro Island (Figure 1; Tanduyan et al., 2013).
Pacijan Island is characterized as grassy with muddy substrates, dominated by Rhizophora, Avicennia, and Sonneratia species. Poro Island is characterized as muddy with rocky substrates, dominated also by Rhizophora, Avicennia, and Sonneratia species. While Ponson Island is characterized as sandy with muddy substrates, dominated by Avicennia, and Sonneratia species (Figure 2; Tanduyan et al., 2013). Mangrove forest is found intact in the coastal areas of Camotes Island.
Methods of Gathering the data
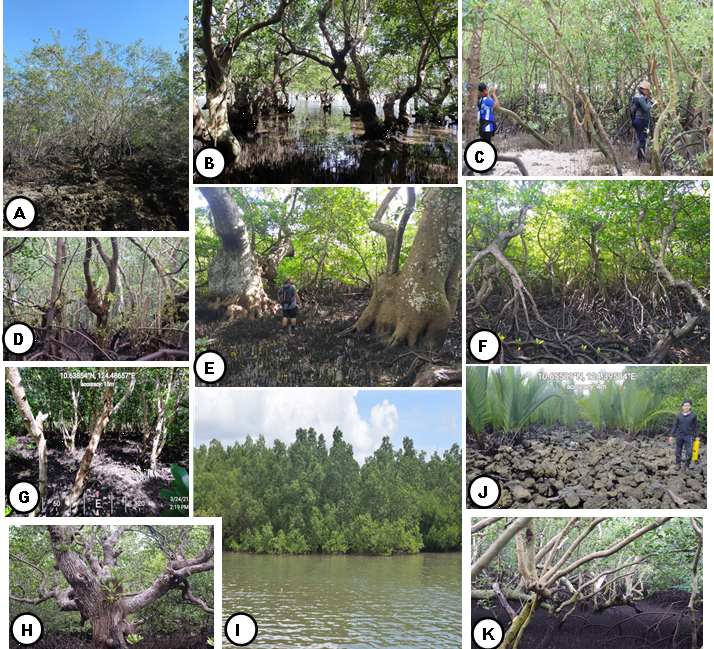
A Belt in line transect method was used in the assessment of mangrove species in the site. Three line transects with a minimum length of 1000m and a maximum of 2000m were established (landward, middle, and seaward) in each sampling sites at an interval of 100m. Quadrats of 10 x 10m dimension were then established within the line transect, at an interval of 250m, to a total of 108 plots/quadrats covering all sampling sites.
All the plant species within the quadrats were recorded and identified following the nomenclature of Primavera and Sadaba (2012). The sample specimens was verified also from manuals, herbarium, Co-digital flora of the Philippines, and online literature for accurate identification of the species. Conservation status of the species were based on Department of Environment and Natural Resources Red list of threatened species (DENR-DAO, 2017-11), as well as on IUCN red list (IUCN, 2021-2).
Trees with diameter of 10cm and above were measured in terms of their diameter breast height (DBH) in centimeter (cm), and total height in meter (m). The measurement of DBH was done with the use of diameter tape for larger trees, and tree caliper for smaller trees. For the total height of the trees, the measurement was made by using an Abney hand level. Basal area (m2) computation for the relative dominance computation was based on DENR formula (Basal Area = 0.7854 D2).
Human-related disturbances to mangrove forest were assessed through actual observation by the researchers. Disturbances observed were verified using the Focused Group Discussion (FGD), and the Key Informant Interview (KII) to the coastal residents in the area.
Data Analysis
Diversity of species. Species diversity were computed and interpreted by using the Shannon, and Simpson Diversity Index. The Multi-Variant Statistical Package (MVSP) software was used to compute the Shannon (H’) and Simpson Diversity Index.
Species Density, Dominance, Frequency, and Importance Value Index (IVI). All recorded data was stored in a Microsoft Excel database and analyzed quantitatively by using Microsoft Excel statistics. Vegetation analysis was performed using the formula of Density, Relative Density, Dominance based on Basal Area, Relative Dominance, Frequency, Relative Frequency, and Importance Value Index (IVI). The ecological importance of each species in relation to the total forest community was calculated by summing its Relative Density, Relative Dominance, and Relative Frequency.
Clustering of native tree species community. Differences in mangrove species composition between sites were assessed with floristic dissimilarity matrices in terms of presence/absence (PRAB) and species abundance (ABU) data criteria. Clustering analysis of mangrove species community and composition were determined using the Jaccard’s matrix, and Sorensen dissimilarity matrix through the MVSP software.
Result and Discussion
Species composition
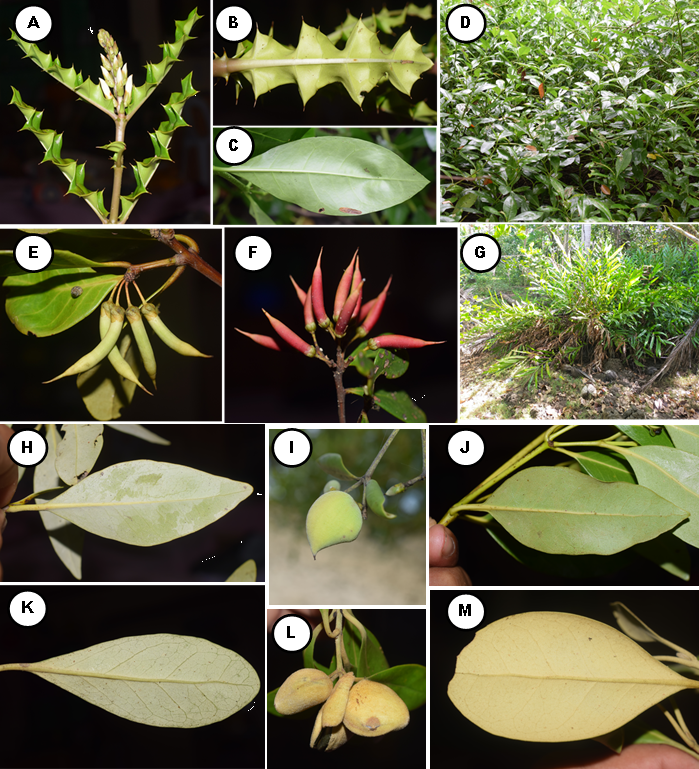
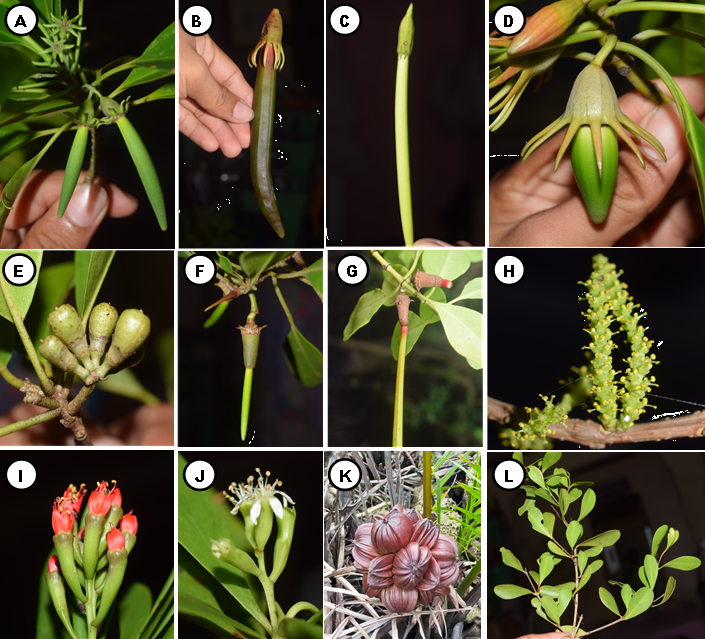
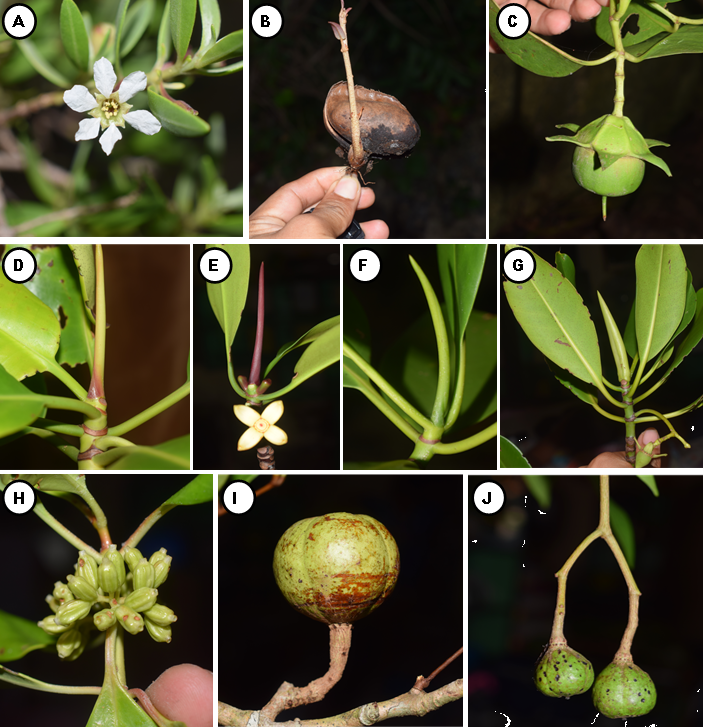
A total of 42 mangrove species recorded in Camotes Island. Out of 42 species, 31 were categorized as true mangrove, and 11 were mangrove associates (Table 1; Figure 3, 4, and 5). The 31 true mangrove species were classified into 13 families and 17 genera (Table 1; Figure 3, 4, and 5). Of the 31 true mangrove species, 27 were recorded in Poro, 26 in Pilar, and 25 for both Tudela and San Francisco sampling sites (Table 1). Camptostemon philippinense (S.Vidal) Becc species was one of the identified threatened species in the island, recorded only in Poro and Pilar sampling sites, together with Scyphiphora hydrophylacea C.F.Gaertn and Avicennia alba Blume making them more diverse as compared to other sampling sites. The 11 mangrove associate species were recorded in all sampling sites. Rhizopohoraceae was the most recorded Family, and Rhizophora was the most dominant genus. The dominant species was Sonneratia alba Sm and recorded in almost all sampling sites. Sonneratia alba Sm species was recorded in the seaward side of all the sampling sites. The result of the study compliment to the findings of Reef and Lovelock (2014), that Sonneratia alba Sm together with Rhizophora apiculata and Avicennia marina are the species that grow in ecotypes with medium to higher salinities such as in estuarine and seaward zones.
Mangrove biologists and experts classify mangrove plants into true mangrove and mangrove associates species. True mangrove limited only to the species that thrive in mangrove habitat, while mangrove associates species distributed in a terrestrial habitat, but found also occurring in the mangrove ecosystem (Polidoro et al., 2010).
The recorded 31 true mangrove species in Camotes Island constituted to 79.5% of the 39 true mangrove species recorded in the Philippines (Sinfuego and Bout, 2014). The result was also higher as compared to both Palawan Island with 23 true mangrove species (Dangan-Galon, et al. 2016), and Dinagat Island with 10 species, 8 families and 9 genera (Lillo and Fernando, 2017). The result implies that Camotes Island serve as a home or habitat to a diverse mangrove species.
| Scientific name | Family name | Sampling sites | |||
|---|---|---|---|---|---|
| San Francisco | Poro | Tudela | Pilar | ||
| True Mangrove Species | |||||
| Acanthus ebracteatus Vahl | Acanthaceae | / | / | / | |
| Acanthus volubilis Wall | Acanthaceae | / | |||
| Aegiceras corniculatum (L.) Blanco | Primulaceae | / | / | / | |
| Aegiceras floridum Roem. & Schult | Primulaceae | / | / | / | / |
| Avicennia alba Blume | Acanthaceae | / | / | ||
| Avicennia marina (Forssk.) Vierh | Acanthaceae | / | / | / | / |
| Avicennia officinalis L. | Acanthaceae | / | / | / | / |
| Avicennia rumphiana Hallier f. | Acanthaceae | / | / | / | / |
| Bruguiera cylindrica (L.) Blume | Rhizophoraceae | / | / | / | / |
| Bruguiera gymnorhiza (L.) Savigny in Lam | Rhizophoraceae | / | / | / | / |
| Bruguiera parviflora (Roxb.) Wight & Arn. ex Griff | Rhizophoraceae | / | / | ||
| Bruguiera sexangula (Lour.) Poir | Rhizophoraceae | / | / | ||
| Camptostemon philippinense (S.Vidal) Becc | Bombacaceae | / | / | ||
| Ceriops tagal (Perr.) C.B.Rob | Rhizophoraceae | / | / | / | / |
| Ceriops zippeliana Blume | Rhizophoraceae | / | / | / | / |
| Excoecaria agallocha L., Syst | Euphorbiaceae | / | / | / | / |
| Heritiera littoralisDryand. inAiton | Sterculiaceae | / | / | / | / |
| Lumnitzera littorea (Jack) Voigt | Combretaceae | / | / | ||
| Lumnitzera racemosa Willd | Combretaceae | / | / | / | / |
| Nypa fruticans Wurmb | Arecaceae | / | / | / | / |
| Osbornia octodonta F. Muell. | Myrtaceae | / | / | / | / |
| Pemphis acidula J.R.Forst. & G.Forst | Lythraceae | / | / | / | |
| Rhizophora apiculata Blume | Rhizophoraceae | / | / | / | / |
| Rhizophora lamarckii Montrouz | Rhizophoraceae | / | / | / | / |
| Rhizophora mucronata Poir. | Rhizophoraceae | / | / | / | / |
| Rhizophora stylosa Griff. | Rhizophoraceae | / | / | / | / |
| Scyphiphora hydrophylacea C.F.Gaertn | Rubiaceae | / | / | ||
| Sonneratia alba Sm. | Lythraceae | / | / | / | / |
| Xylocarpus granatum Koen | Meliaceae | / | / | / | / |
| Xylocarpus moluccensis (Lam.) M.Roem | Meliaceae | / | / | / | / |
| Acrostichum speciosum Willd | Pteridaceae | / | |||
| Sub-Total | 25 | 27 | 25 | 26 | |
| Mangrove associate species | |||||
| Hoya sp. | Apocynaceae | / | / | / | / |
| Terminalia catappa Linn | Combretaceae | / | / | / | / |
| Glochidion littorale Blume | Phyllanthaceae | / | / | / | / |
| Breynia vitis-idaea (Burm.f.) C.E.C..Fischer | Phyllanthaceae | / | / | / | / |
| Abrus precatorius Linn. | Fabaceae | / | / | / | / |
| Albizia retusa Benth. | Fabaceae | / | / | / | / |
| Caesalpinia crista Linn. | Fabaceae | / | / | / | / |
| Derris trifoliate Lour. | Fabaceae | / | / | / | / |
| Flagellaria indica Linn. | Flagellariaceae | / | / | / | / |
| Talipariti tiliaceum (Linn.) Fryxell | Malvaceae | / | / | / | / |
| Morinda citrifolia Linn | Rubiaceae | / | / | / | / |
| Sub-Total | 11 | 11 | 11 | 11 | |
| Grand total | 36 | 38 | 36 | 37 | |
Diversity of species
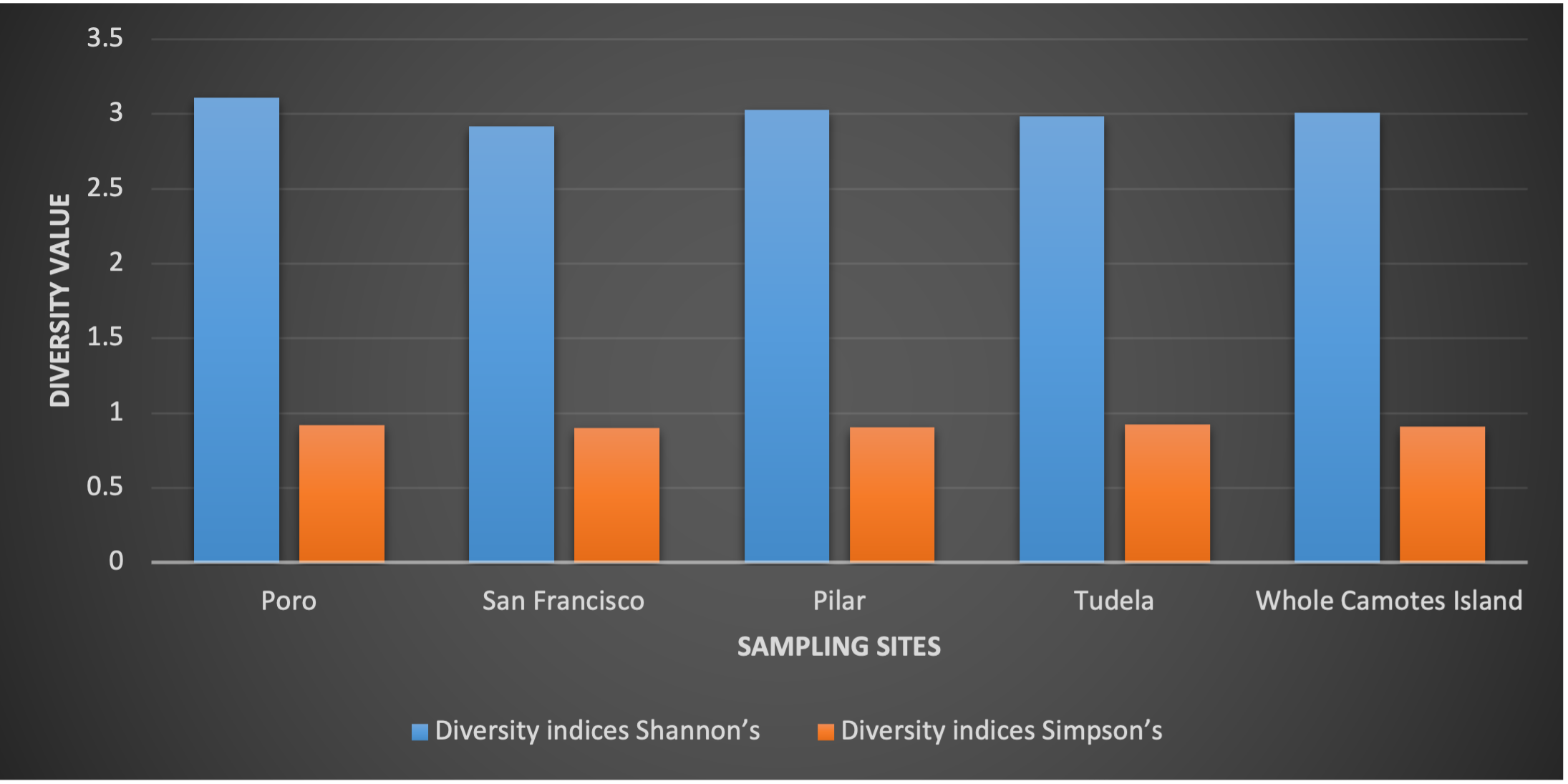
Based from the computed diversity index by Shannon, Camotes Island has a species diversity of H’=3.0107 (Figure 6), with a relative value of high species diversity (MacDonald 2003). The result of the study compliment with the total number of true mangrove species identified in the island with 31 (Table 1).
Among the sampling sites Poro has the highest in terms of computed diversity value of H’=3.111, followed by Pilar with a computed diversity value of H’=3.027 (Figure 6). Both Poro and Pilar sampling sites have a relative value of high species diversity (MacDonald, 2003). The result compliment to the number of identified true mangrove species in both sampling site (Table 1).
San Francisco and Tudela sampling sites both have a computed diversity value of H’=2.9 (Figure 6). The sampling sites have a relative value of moderately high species diversity (MacDonald, 2003). The result compliment to the number of identified true mangrove species in both sampling sites with lower in number as compared to Poro and Pilar (Table 1).
In Simpson’s diversity index, all the sampling sites have a relative value of high species diversity (Figure 6). The result of the study implies that the mangrove forest of Camotes Island was still stable and productive. Lillo et al. (2019) emphasized that Species diversity of a certain community attributed to its stability, productivity, and trophic structure. An area with high species diversity results to a more stable and productive ecosystem.
However, as observed in the different sampling sites, anthropogenic activities were existing, and causing damage to mangrove forest, as well as threaten the mangrove species. This anthropogenic activities include illegal cutting, and according to the coastal communities interviewed, they used it for fuel wood production and for charcoal making. Other activities include gathering of leaves and branches for forage, improper disposal of garbage, and debarking of mangrove trees for tan bark production, as well as reclamation of the coastal areas for community and industrial development.
The uncontrolled anthropogenic activities threaten the stability and productivity of the whole Camotes Island mangrove forest. The different Local Government Units in the area must strictly implement the policy of protecting the mangrove species, since the whole Camotes Island was declared as protected area under Proclamation No. 2152, s. 1981.
Species Density, Dominance, Frequency and Importance value Index (IVI)
The mangrove species with higher in Importance Value Index (IVI) in Camotes Island was Sonneratia alba Sm. (39.17%) (Table 2). The species was the most dominant in terms of diameter, richness, and density in all sampling sites. Based on actual measurement the species reached a maximum diameter of 80cm and total height of 15m. Saplings and wildlings of the species were also abundant in the forest floor in all sampling sites. Identification of the most dominant mangrove species in a particular ecotype provide insights as to what species could be used for the future rehabilitation programs in a certain mangrove ecosystems (Raganas and Magcale-Macandog, 2020).
Other mangrove species with higher Importance Value Index were Avicennia marina (Forssk.) Vierh (26.13%), Avicennia rumphiana Hallier f, ( 24.57%), Rhizophora apiculata Blume, (23.05%), Rhizophora stylosa Griff., (15.82%), Avicennia officinalis L. (12.93%), Ceriops zippeliana Blume, (11.48%), and Xylocarpus granatum Koen. (10.15%). The species were dominant in terms of frequency. However, they have a smaller in diameter as compared to Sonneratia alba Sm. The diameter of the trees ranges from 10–40cm, and total height ranges from 8–15m.
In Tudela, Poro, and San Francisco sampling sites Sonneratia alba Sm was also considered as the species with higher Importance Value Index (Table 2; Figure 2). The species was also dominant in terms of diameter, frequency, and density. Other dominant species were Avicennia marina (Forssk.) Vierh, Rhizophora apiculata Blume, Rhizophora stylosa Griff, Ceriops zippeliana Blume, Xylocarpus granatum Koen, and Avicennia rumphiana Hallier f. (Table 2).
However, in Pilar sampling site, Rhizophora apiculata Blume was considered as the species with higher Importance Value Index (Table 2). The species was dominant in terms of frequency in all sampling plots. The species has a diameter ranges from 10–40 cm, smaller as compared to Sonneratia alba Sm. The total height ranges from 10–15m, similar to Sonneratia alba Sm. Other dominant species were Avicennia marina (Forssk.) Vierh, Sonneratia alba Sm., Rhizophora stylosa Griff, Ceriops zippeliana Blume, Avicennia rumphiana Hallier f, and Rhizophora mucronata Poir. (Table 2). The forest floor of the mangrove forest was also dominated by the seedlings and saplings of the species of Rhizophora apiculata Blume, Rhizophora mucronata Lam, Rhizophora stylosa Griff., Sonneratia alba Sm, and Avicennia marina (Forssk.) Vierh. Rhizophora mucronata Poir was also dominant in Poro and San Francisco sampling sites. But the species was not dominant in Tudela sampling site.
The result of the study is similar with Palawan Island mangrove forest which are dominated by the species of Lumnitzera littorea, Rhizophora apiculata, Rhizophora mucronata, Rhizophora stylosa, Scyphiphora hydrophyllacea, and Sonneratia alba (Dangan-Galon et al., 2016). Similar species are also found dominant in Dinagat Island mangrove forest (Lillo and Fernando, 2017). Das et al. (2019) emphasizing that the distribution pattern of the species have been linked with the Salinity, sulfides, nutrients, sedimentation, soil texture, and nutrients of a certain mangrove ecosystem.
| Species | Sampling sites | Rel. Den. | Rel. Dom. | Rel. Freq. | IVI | |||
|---|---|---|---|---|---|---|---|---|
| Pilar | Poro | San Fran | Tudela | |||||
| Sonneratia alba Sm. | 183 | 193 | 138 | 161 | 26.29 | 8.29 | 4.60 | 39.17 |
| Avicennia marina (Forssk.) Vierh | 186 | 105 | 77 | 96 | 18.07 | 3.46 | 4.60 | 26.13 |
| Avicennia rumphiana Hallier f | 39 | 17 | 12 | 17 | 3.31 | 16.67 | 4.60 | 24.57 |
| Rhizophora apiculata Blume | 207 | 77 | 107 | 45 | 16.98 | 1.48 | 4.60 | 23.05 |
| Rhizophora stylosa Griff. | 99 | 31 | 55 | 83 | 10.44 | 0.78 | 4.60 | 15.82 |
| Avicennia officinalis L. | 13 | 4 | 0 | 0 | 0.66 | 9.97 | 2.30 | 12.93 |
| Ceriops zippeliana Blume | 65 | 47 | 20 | 30 | 6.31 | 0.57 | 4.60 | 11.48 |
| Xylocarpus granatum Koen | 3 | 15 | 1 | 21 | 1.56 | 4.00 | 4.60 | 10.15 |
| Avicennia alba Blume | 2 | 0 | 0 | 0 | 0.08 | 5.11 | 4.60 | 9.79 |
| Heritiera littoralis Aiton | 0 | 2 | 0 | 0 | 0.08 | 5.11 | 4.60 | 9.79 |
| Rhizophora mucronata Poir. | 37 | 13 | 11 | 4 | 2.53 | 0.97 | 4.60 | 8.10 |
| Excoecaria agallocha L. | 5 | 5 | 3 | 16 | 1.13 | 2.36 | 4.60 | 8.08 |
| Bruguiera cylindrica (L.) Blume | 7 | 6 | 7 | 18 | 1.48 | 1.99 | 4.60 | 8.06 |
| Xylocarpus rumphii (Kostel.) Mabb | 4 | 13 | 6 | 10 | 1.29 | 2.16 | 4.60 | 8.05 |
| Bruguiera sexangula (Lour.) Poir | 0 | 0 | 0 | 17 | 0.66 | 6.14 | 1.15 | 7.95 |
| Ceriops tagal (Perr.) C.B.Rob | 7 | 19 | 18 | 6 | 1.95 | 0.93 | 4.60 | 7.48 |
| Rhizophora lamarckii Montrouz | 15 | 13 | 7 | 5 | 1.56 | 0.91 | 4.60 | 7.07 |
| Bruguiera gymnorhiza (L.) Savigny in Lam | 4 | 7 | 3 | 1 | 0.58 | 1.28 | 4.60 | 6.46 |
| Lumnitzera racemosa Willd | 1 | 3 | 7 | 8 | 0.74 | 0.81 | 4.60 | 6.15 |
| Osbornia octodonta F. Muell. | 1 | 17 | 0 | 10 | 1.09 | 1.05 | 3.45 | 5.59 |
| Aegiceras floridum Roem. & Schult | 12 | 9 | 0 | 6 | 1.05 | 0.99 | 3.45 | 5.49 |
| Aegiceras corniculatum (L.) Blanco | 0 | 7 | 1 | 2 | 0.39 | 1.60 | 3.45 | 5.44 |
| Camptostemon philippinense (S.Vidal) Becc | 5 | 3 | 0 | 0 | 0.31 | 1.84 | 2.30 | 4.45 |
| Pemphis acidula J.R.Forst. & G.Forst | 0 | 10 | 0 | 3 | 0.51 | 1.63 | 2.30 | 4.43 |
| Bruguiera parviflora (Roxb.) Wight & Arn. ex Griff | 1 | 0 | 1 | 0 | 0.08 | 1.34 | 2.30 | 3.71 |
| Scyphiphora hydrophylacea C.F.Gaertn | 1 | 3 | 0 | 0 | 0.16 | 1.22 | 2.30 | 3.68 |
| Lumnitzera littorea (Jack) Voigt | 0 | 0 | 0 | 18 | 0.70 | 1.63 | 1.15 | 3.49 |
| Prosopis juliflora (Sw.) DC | 0 | 0 | 1 | 0 | 0.04 | 1.71 | 1.15 | 2.90 |
Clustering of Mangrove species (Jaccard’s, and Sorensen Dissimilarity Matrix)
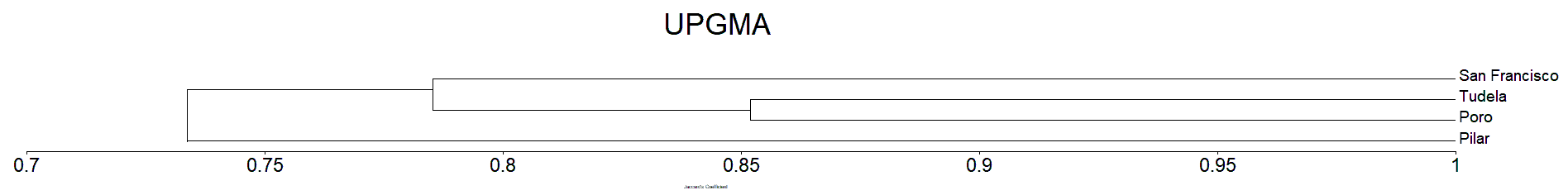
Clustering analysis of the Mangrove species in Camotes Island by Jaccard’s dissimilarity matrix shows that all the plots sampled were forming into three clusters/groups correspondingly (Figure 7). The four sampling sites from Camotes Island proved to have distinctive mangrove species association, hence grouping them together into three distinct clusters. Tudela and Poro sampling sites formed as one group, while San Francisco sampling site formed as one cluster distinct from other sampling sites. Pilar sampling site also formed as one cluster similar to San Francisco. The species composition of the site was distinct from other sampling sites.
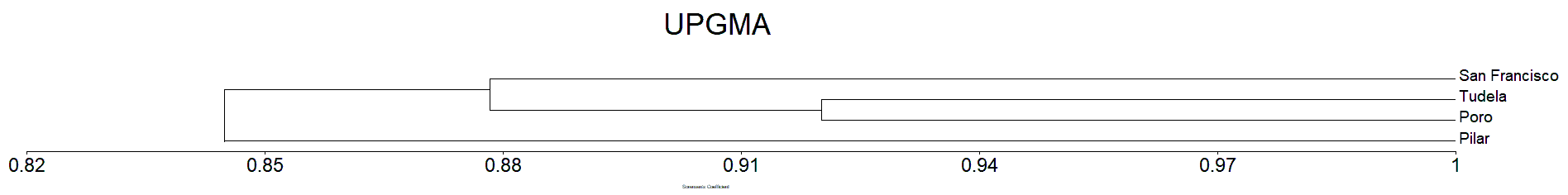
The Sorensen dissimilarity matrix shows that all the plots sampled were forming into three clusters/groups similar result in Jaccard’s (Figure 7 and 8). Both the Jaccard’s and Sorensen dissimilarity matrix prove that the mangrove species of Camotes Island were forming three cluster. Wherein the species in San Francisco form as one cluster, similar with Pilar sampling site. While Poro and Tudela mangrove species found to be similar from each other, together forming as one cluster (Figure 7 and 8).
The mangrove species in San Francisco sampling site formed as one cluster because it differ in environmental condition particularly on its substrate from the other sampling sites. Similar situation with Pilar sampling sites. The mangrove species in Poro and Tudela sampling sites was more likely similar from each other, forming as one cluster together because they have similar substrate (Tanduyan et al., 2013).
The result of the study compliment with the geographical location of the different major islands of Camotes Island (Figure 1). The results were also supported by Raganas and Magcale-Macandog (2020) that the differences in the mangrove environmental conditions can result in the dominance of a particular species leading to their habitat differentiation.
Conservation Status of the species
Of the 31 true mangrove species, two were identified as threatened and categorized as Endangered species under DENR national listing (DAO, 2017-11). The species include Pemphis acidula J.R.Forst. & G.Forst, and Camptostemon philippinense (S.Vidal) Becc which were also considered as native species to the Philippines. In IUCN red list (IUCN, 2021-2), three species were found to be threatened, and categorized as Endangered (Camptostemon philippinense (S.Vidal) Becc), Vulnerable (Avicennia rumphiana Hallier f.), and Near Threatened (Aegiceras floridum Roem. & Schult). Pemphis acidula J.R.Forst. & G.Forst species was categorized as Endangered by DENR (DAO, 2017-11), but in IUCN (IUCN, 2021-2) the species was still in Least Concern category (Table 3). Then Avicennia rumphiana Hallier f. and Aegiceras floridum Roem species were categorized as threatened in IUCN (IUCN, 2021-2), but in DENR (DAO, 2017-11) the species were now categorized as not yet assessed. As observed in the sampling sites, the two species were still dominant in the wild.
Villanueva and Buot (2015) reiterating that in updating the conservation status of an individual species are actually based on the area and species priority of an accrediting institution. Accrediting institution with larger area of coverage could hardly update its record regularly, as compared to a local accrediting institution.
| Species | Family name | Conservation Status | ||
|---|---|---|---|---|
| Scientific name | Common name | DAO 2017-11 | IUCN 2021-2 | |
| Acanthus ebracteatus Vahl | Lagiwliw | Acanthaceae | Not yet assessed | Least concern |
| Acanthus volubilis Wall | Lagiwliw | Acanthaceae | Not yet assessed | Least concern |
| Aegiceras corniculatum (L.) Blanco | Saging2x | Primulaceae | Not yet assessed | Least concern |
| Aegiceras floridum Roem. & Schult | Saging2x pula | Primulaceae | Not yet assessed | Near Threatened |
| Avicennia alba Blume | Api-api | Acanthaceae | Not yet assessed | Least concern |
| Avicennia marina (Forssk.) Vierh | Miapi | Acanthaceae | Not yet assessed | Least concern |
| Avicennia officinalis L. | Bungalon | Acanthaceae | Not yet assessed | Least concern |
| Avicennia rumphiana Hallier f. | Bungalon | Acanthaceae | Not yet assessed | Vulnerable |
| Bruguiera cylindrica (L.) Blume | Pototan | Rhizophoraceae | Not yet assessed | Least concern |
| Bruguiera gymnorhiza (L.) Savigny in Lam | Busain | Rhizophoraceae | Not yet assessed | Least concern |
| Bruguiera parviflora (Roxb.) Wight & Arn. ex Griff | Langarai | Rhizophoraceae | Not yet assessed | Least concern |
| Bruguiera sexangula (Lour.) Poir | Malatalong | Rhizophoraceae | Not yet assessed | Least concern |
| Camptostemon philippinense (S.Vidal) Becc | Gapas2x | Bombacaceae | Endangered | Endangered |
| Ceriops tagal (Perr.) C.B.Rob | Tungog | Rhizophoraceae | Not yet assessed | Least concern |
| Ceriops zippeliana Blume | Lapis2x | Rhizophoraceae | Not yet assessed | Least concern |
| Excoecaria agallocha L., Syst | Buta2x | Euphorbiaceae | Not yet assessed | Least concern |
| Lumnitzera littorea (Jack) Voigt | Tabao pula | Combretaceae | Not yet assessed | Least concern |
| Lumnitzera racemosa Willd | Culase | Combretaceae | Not yet assessed | Least concern |
| Nypa fruticans Wurmb | Nipa | Arecaceae | Not yet assessed | Least concern |
| Osbornia octodonta F. Muell. | Tawalis | Myrtaceae | Not yet assessed | Least concern |
| Pemphis acidula J.R.Forst. & G.Forst | Bantigi | Lythraceae | Endangered | Least concern |
| Rhizophora apiculata Blume | Bakhaw lalaki | Rhizophoraceae | Not yet assessed | Least concern |
| Rhizophora lamarckii Montrouz | Bakhaw hybrid | Rhizophoraceae | Not yet assessed | Not evaluate |
| Rhizophora mucronata Poir. | Bakhaw babae | Rhizophoraceae | Not yet assessed | Least concern |
| Rhizophora stylosa Griff. | Bakhaw bato | Rhizophoraceae | Not yet assessed | Least concern |
| Scyphiphora hydrophylacea C.F.Gaertn | Nilad | Rubiaceae | Not yet assessed | Least concern |
| Sonneratia alba Sm. | Pagatpat | Lythraceae | Not yet assessed | Least concern |
| Xylocarpus granatum Koen | Tabigi | Meliaceae | Not yet assessed | Least concern |
| Xylocarpus moluccensis (Lam.) M.Roem | Lagutlot | Meliaceae | Not yet assessed | Least concern |
| Acrostichum speciosum Willd | Palaypay | Pteridaceae | Not yet assessed | Least concern |
Conclusion
Conservation effort and Information Education Campaign (IEC) are needed by the Local Government Units to protect the high mangrove species diversity of Camotes Island. The Local Government Unit concerned must strictly implement the policy of protecting the mangrove species, since the whole Camotes Island was declared as protected area under Proclamation No. 2152, s. 1981. Empowerment of the local communities by legitimizing their resource use rights, as well as management responsibilities is the key factor in driving successful mangrove restoration (Camacho et al., 2020).
Acknowledgements
The authors would like to acknowledge the Department of Science and Technology (DOST) for considering and approving their research proposal. The Philippine Consortium for Agriculture and Resource Research Development (PCAARRD) for recommending their proposal to DOST and guiding them in the implementation of the study. The CTU System and CTU Camotes Campus for supporting the research team, and allowing the research staff to conduct the study in Camotes Island. The DENR Region 7 for giving them the Gratuitous permit (GP). The Municipalities of San Francisco, Poro, Pilar, and Tudela for allowing them to conduct the study in their area of responsibilities. The first author (EPL) would like to thanks his wife (Mary Jane) and son (CJ) for their moral support, during the conduct of the study.
References
- Aksornkoae, S., & Kato, S., 2011. Mangroves for the people and environmental conservation in Asia. Bulletin of the Society of Sea Water Science, Japan., 65 (1), 3-9. https://www.jstage.jst.go.jp/article/swsj/65/1/65_3/_pdf
- Camacho, L, Gevaña, D., Carandang, A., Camacho, S., Combalicer, E., Rebugio, L., Youn, Y., Tree biomass and carbon stock of a community-managed mangrove forest in Bohol, Philippines. Forest Sci Tech 7(4):161–167. DOI: 10.1080/21580103.2011.621377
- Camacho, L.D., Gevaña, D.T., Sabino, L.L., Ruzol, C.D., Garcia, J.E., Camacho, A.C.D., Oo, T.N., Maung, A.C., Saxena, K.G., Liang, L., Yiu, E., and Takeuchi, K., 2020. Sustainable mangrove rehabilitation: Lessons and insights from community-based management in the Philippines and Myanmar. DOI: 10.30852/sb.2020.983. https://www.researchgate.net/publication/339792382_Sustainable_mangrove_rehabilitation_Lessons_and_insights_from_community-based_management_in_the_Philippines_and_Myanmar
- Dangan-Galon, F., Roger G. Dolorosa, R.G., Jeter S. Sespen, J.S., Nelly I. Mendoza, N.I., 2016. Diversity and structural complexity of mangrove forest along Puerto Princesa Bay, Palawan Island, Philippines. Journal of Marine and Island Cultures Volume 5, Issue 2, Pages 118-125. DOI: 10.1016/j.imic.2016.09.001
- DAO, 2017. Denr Administrative Order, Updated National List of Threatened Philippine Plants and their Categories (DAO No. 2017-11). https://bmb.gov.ph/index.php/resources/downloadables/laws-and-policies/denr-administrative-orders/dao-2017-2022/file/197-denr-administrative-order-2017-11
- Das, L., Patel, R., Salvi, H., Kamboj, R.D., 2019. Assessment of natural regeneration of mangrove with reference to edaphic factors and water in Southern Gulf of Kachchh, Gujarat, India. Heliyon 5 (8): e02250. DOI: 10.1016/j.heliyon.2019.e02250
- DENR NGP Website. http://ngp.denr.gov.ph/index.php/basic-configuration/site-Administrator/ngp-accomplishment-report
- Doydee P., Buot I.E. Jr., 2010, Mangrove habitat restoration and management in Ranong Coastal Environments, Thailand. https://www.researchgate.net/publication/267827162_MANGROVE_HABITAT_RESTORATION_AND_MANAGEMENT_IN_RANONG_COASTAL_ENVIRONMENTS_THAILAND
- (FAO-UN) Food and Agriculture Organization of the United Nations, 2015. Global forest resources assessment 2015: Desk reference. Rome: Food and Agriculture Organization of the United Nations. http://www.fao.org/3/i4808e/i4808e.pdf
- Garcia, K., Malabrigo, P., & Gevaña, D., 2014. Philippines’ mangrove ecosystem: status, threats and conservation. In Mangrove Ecosystems of Asia (pp. 81-94). New York: Springer. DOI: 10.1007/978-1-4614-8582-7_5
- Gevaña, D., Camacho, L.D., & Pulhin, J.M., 2018. Conserving Mangroves for Their Blue Carbon: Insights and Prospects for Community-Based Mangrove Management in Southeast Asia. DOI: 10.1007/978-3-319-73016-5_26. Springer International Publishing AG, part of Springer Nature 2018. https://www.apn-gcr.org/bulletin/article/sustainable-mangrove-rehabilitation-lessons-and-insights-from-community-based-management-in-the-philippines-and-myanmar/#Gevana2018
- Gevaña, D., Pulhin, J., & Tapia, M., 2019. Fostering climate change mitigation through a community-based approach: Carbon stock potential of community-managed mangroves in the Philippines. In Krishnamurthy, Jonathan, Srinivasalu, Glaeser (Eds.), Coastal Management: Global Challenges and Innovations (pp. 271-282). Elsevier. DOI: 10.1016/B978-0-12-810473-6.00014-5.
- Giri, C., Ochieng, E., Tieszen, L.L., Zhu, Z., Singh, A., Loveland, T., Masek, J., Duke, N., 2010. Status and distribution of mangrove forests of the world using earth observation satellite data, Global Ecology and Biogeography 20: 154-159. http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.694.9931&rep=rep1&type=pdf DOI: 10.1111/j.1466-8238.2010.00584.x
- Hansen, M.C. et al., 2013. High-resolution global maps of 21st-century forest cover change. Science 342: 850–853. DOI: 10.1126/science.1244693
- IUCN., 2021. International Union for Conservation of Nature. IUCN updates 'red list' of endangered species. IUCN 2021-2. https://www.iucn.nl/en/solutions/red-list-of-threatened-species.
- Lillo, E.P., and Buot, I.E. Jr., 2016. Species Composition of Argao Mangrove Forest, Cebu, Philippines. J. Wetlands Biodiversity (2016) 6: 37-45. https://www.muzeulbrailei.ro/images/naturale/Volum%206/04JWB201663745.pdf
- Lillo, E.P., and Edwino S. Fernando, E.S., 2017. Composition and Diversity of Mangrove Species on Dinagat Island, Philippines. J. Wetlands Biodiversity (2017) 7: 91-108. https://www.muzeulbrailei.ro/images/naturale/Volum%207/DeCorectat/07JWB20170791108.pdf
- Lillo, E.P., Malaki, A.B., Alcazar, S.M.T., Nuevo, R.U., and Rosales, R., 2019. Native Trees on Mount Lantoy Key Biodiversity Areas (KBA), Argao, Cebu, Philippines. Philippine Journal of Science 148 (2): 359-371, June 2019 ISSN 0031-7683. https://philjournalsci.dost.gov.ph/images/pdf/pjs_pdf/vol148no2/native_trees_on_mt_lantoy_.pdf
- Macdonald, G.M., 2003. Biogeography: Space, Time, and Life. New York: John Wiley & Sons, Inc. 528p.
- Maritime Review., 2017. Mangrove Forests in the Philippines. https://maritimereview.ph/mangrove-forests-in-the-philippines/
- Macintosh, D.J., 2010. Coastal Community Livelihoods: implication of intact ecosystem services. Paper presented at the 2010 Katoomba Meeting XVII held June 23-24, 2010 in Hanoi, Vietnam. http://www.ecosystemmarketplace.com/documents/acrobat/k17/Don%20Macintosh.pdf.
- Philippine Statistics Authority., 2017. Census of population 2015. Philippine Statistics Authority. https://psa.gov.ph/statistics/census/2015-census-of-population
- Polidoro, B.A., Carpenter, K.E., Collins, L., Duke, N.C., Ellison, A.M., Ellison, J.C, et al., 2010. The Loss of Species: Mangrove Extinction Risk and Geographic Areas of Global Concern. PLoS ONE 5(4): e10095. DOI: 10.1371/journal.pone.0010095
- Primavera, J. H., & Sadaba, R. B., 2012. Beach forest species and mangrove associates in the Philippines. Aquaculture Department, Southeast Asian Fisheries Development Center. ISBN 9789719931010. http://hdl.handle.net/10862/6045
- Proclamation No. 2152, s., 1981. Declaring the Entire Province of Palawan and Certain Parcels of the Public Domain and/or Parts of the Country as Mangrove Swamp Forest Reserves. https://www.officialgazette.gov.ph/1981/12/29/proclamation-no-2152-s-1981/
- Raganas, A.F.M., and Magcale-Macandog, D.B., 2020.Physicochemical factors influencing zonation patterns, niche width and tolerances of dominant mangroves in southern Oriental Mindoro, Philippines. OCEAN LIFE Volume 4, Number 2, December 2020 E-ISSN: 2580-4529 Pages: 51-62 DOI: 10.13057/oceanlife/o040201
- Reef, R., Lovelock, C.E., 2014. Regulation of water balance in mangroves. Ann Bot 115(3): 385-395.
- Sinfuego, K.S., and Buot, I.E.Jr., 2014. Mangrove zonation and utilization by the local people in Ajuy and Pedada Bays, Panay Island, Philippines. Journal of Marine and Island Cultures Volume 3, Issue 1, June 2014, Pages 1-8. https://doi.org/10.1016/j.imic.2013.11.002
- Tanduyan, S.N., Andriano, B.T., Ciriaco, P.E., Gonzaga, R.B., Anoos, W.G., and Garciano, L.M., Frequently gleaned holothurians in Camotes Islands, Central Philippines. Galaxea, Journal of Coral Reef Studies (Special Issue): 253-259. https://www.jstage.jst.go.jp/article/galaxea/15/Supplement/15_253/_pdf/-char/ja
- Thompson, B., Clubbe, C., Primavera, J., Curnick, D., & Koldewey, H., 2014. Locally assessing the economic viability of blue carbon: A case study from PanayIsland, the Philippines. Ecosystem Services,8, 128-140. DOI: 10.1016/j.ecoser.2014.03.004
- Villanueva ELC, Buot IE Jr., 2015. Threatened plant species of Mindoro, Philippines. IAMURE Intl J Ecol Conserv 14: 168–189. DOI: 10.7718/ijec.v14i1.901
